LIMS: Complete Laboratory Information Management Solution - Revol LIMS
LIMS - Brief Overview
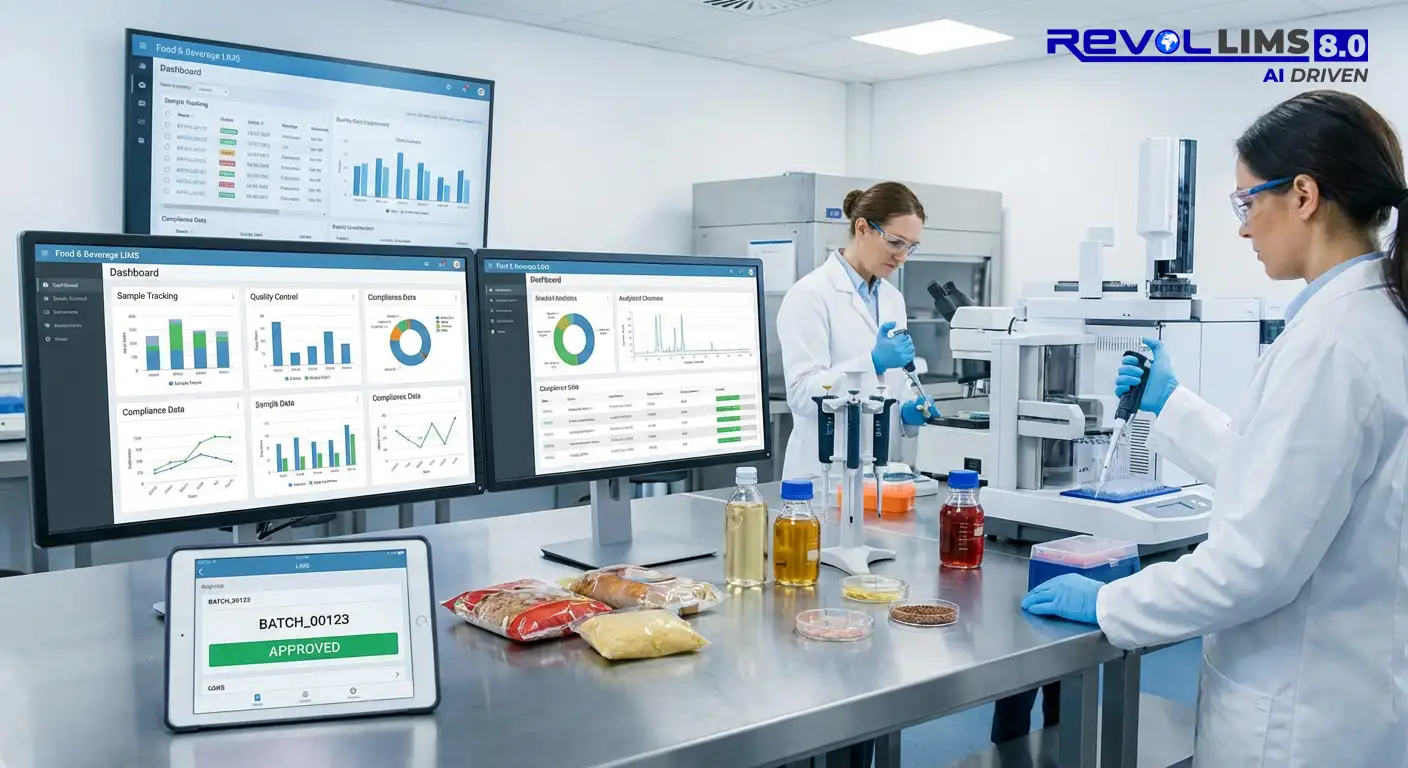
A Laboratory Information Management System (LIMS) is specialised software that manages laboratory operations, samples, workflows, data, and compliance. Revol LIMS goes beyond traditional systems by integrating artificial intelligence, automated workflows, and industry-specific configurations to deliver faster ROI and superior operational efficiency.
Transform Your Laboratory with AI-Powered LIMS Technology
The most advanced Laboratory Information Management System designed for modern laboratories. Achieve operational excellence, regulatory compliance, and data-driven insights with Revol LIMS.
Request Live Demo | View Pricing | Industries We Serve
Why Laboratories Worldwide Choose Revol LIMS:
- 12-Week Implementation vs 6-18 months industry standard
- 40% Lower TCO than legacy LIMS platforms
- AI-Powered Intelligence for predictive analytics
- ISO 17025 & FDA 21 CFR Part 11 compliant out-of-the-box
- 15+ Years of proven expertise
Already know what LIMS is? Jump to Revol LIMS Features →
Need more details? Read our comprehensive LIMS guide →
The Revol LIMS Difference
Industry-Leading Features That Drive Results
Rapid Implementation
Unlike competitors requiring 6-18 months, Revol LIMS deploys in 12 weeks with our proven methodology:
- Pre-configured industry workflows
- Dedicated implementation team
- Comprehensive validation documentation
- On-site go-live support
- Immediate productivity from day one
AI-Driven Laboratory Intelligence
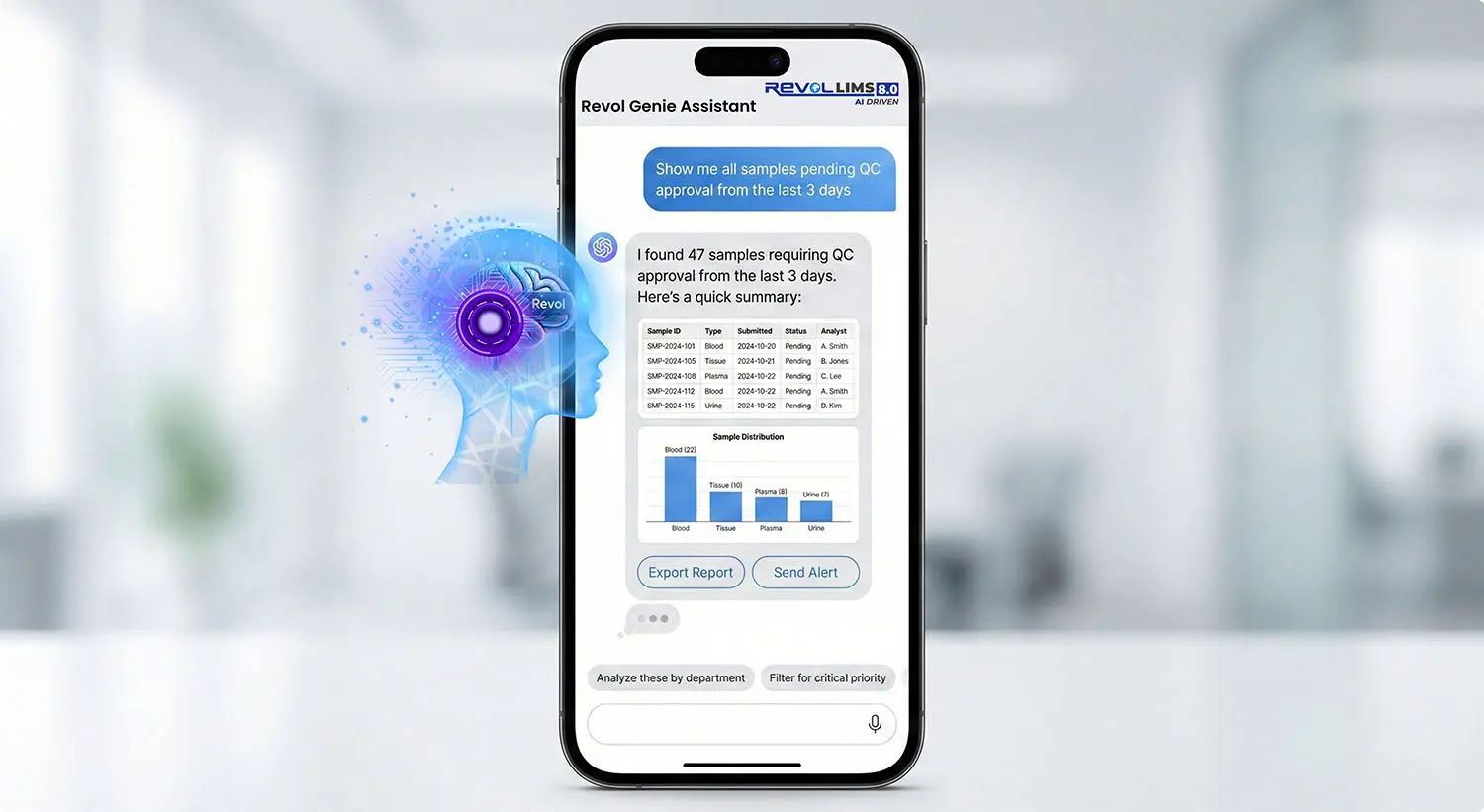
Advanced artificial intelligence transforms your laboratory data:
- Gen-AI Chat Assistant for natural language queries
- Predictive Analytics identifies trends before issues arise
- Automated Anomaly Detection in test results and QC
- Auto Analyst Assignment optimises workflow efficiency
- Real-time SQC/SPC Monitoring with automatic alerts
Transparent, Predictable Pricing
No hidden fees. No surprise costs. 40% lower total cost of ownership includes:
- All-inclusive software licensing
- Complete implementation services
- Unlimited user access
- Comprehensive training programs
- Typical ROI: 12-24 months
Industry-Specific Solutions
Purpose-built for your laboratory type—not generic software requiring extensive customisation:
- Pharmaceutical & Biotech - GMP compliant, stability studies
- Food & Beverage - HACCP support, allergen management
- Environmental Testing - EPA method compliance, field sampling
- Clinical Diagnostics - CLIA compliant, patient workflows
- Petrochemical - ASTM methods, quality assurance
- Cannabis Testing - State compliance, potency analysis
- View All Industry Solutions →
Seamless Integration Ecosystem
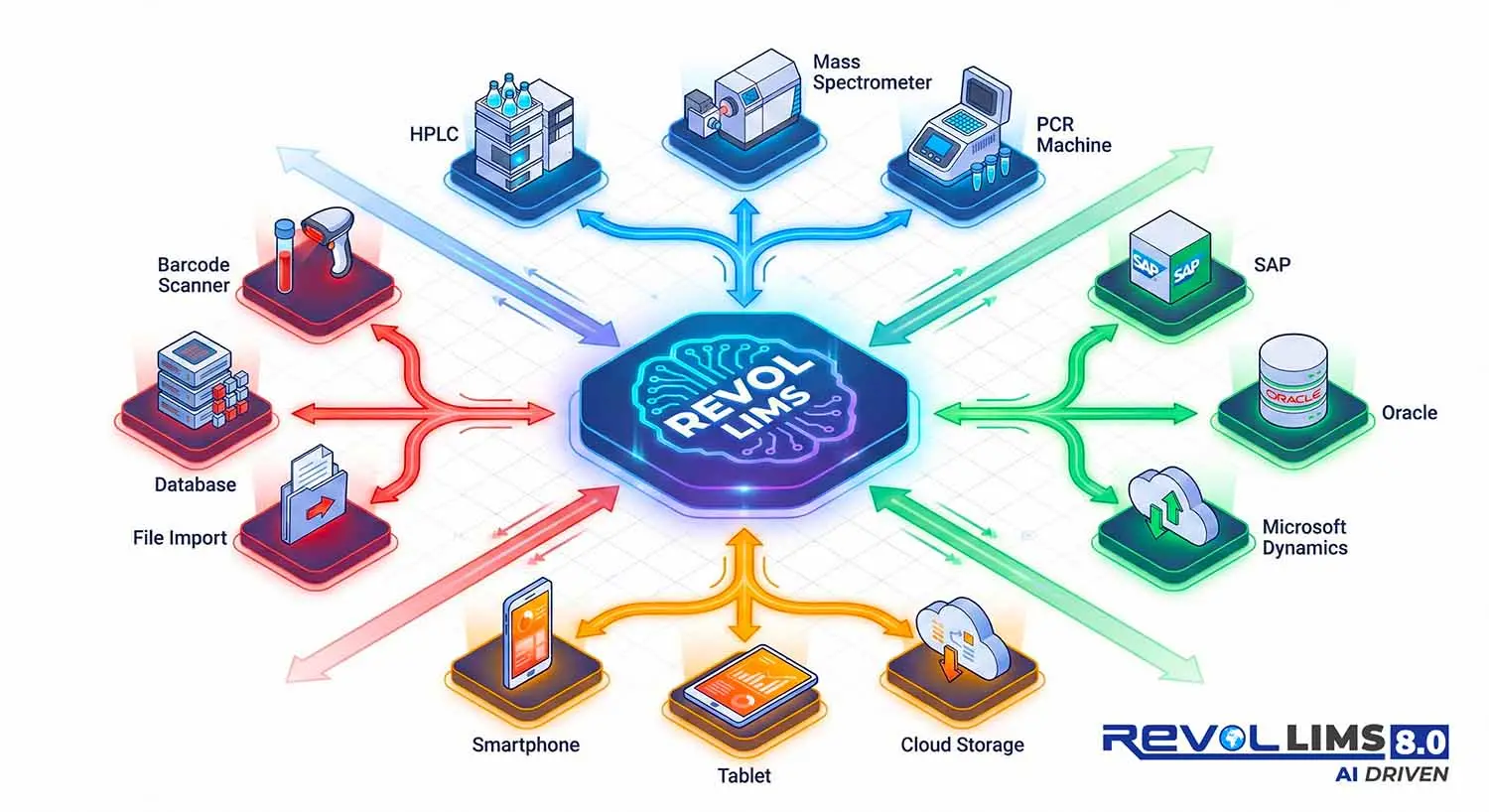
Connect with your existing laboratory infrastructure:
- Direct Instrument Integration - HPLC, mass spec, PCR, spectrophotometers
- ERP Connectivity - SAP, Oracle, Microsoft Dynamics
- API-First Architecture - RESTful APIs for custom integrations
- 100% Web-Based – Cloud or on-premise deployment
- Mobile Application - iOS & Android for field operations
Built-In Compliance
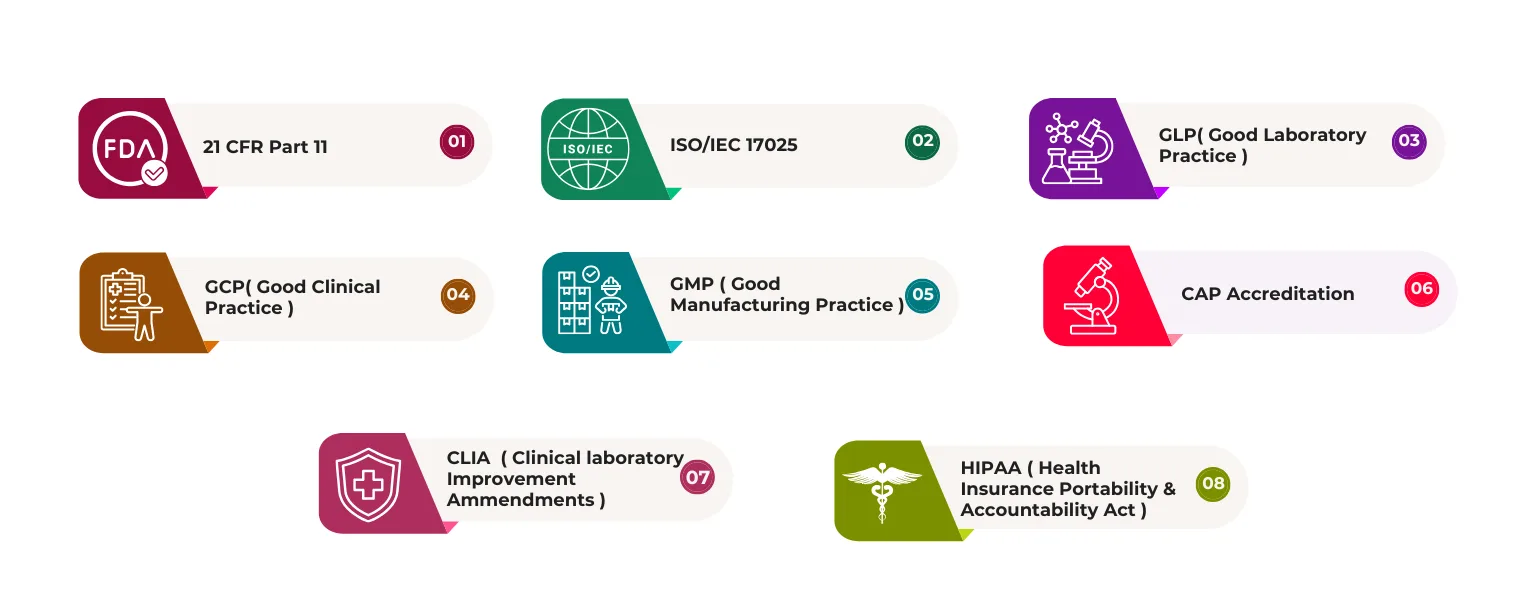
Regulatory compliance is core to our architecture, not an add-on:
- FDA 21 CFR Part 11 - Electronic signatures & audit trails
- ISO/IEC 17025 - Accreditation-ready out-of-the-box
- GLP, GMP, GCP - Pharmaceutical compliance
- CLIA, CAP - Clinical laboratory standards
- HIPAA - Healthcare data protection
- Complete Audit Trails - Every action documented
Core LIMS Capabilities
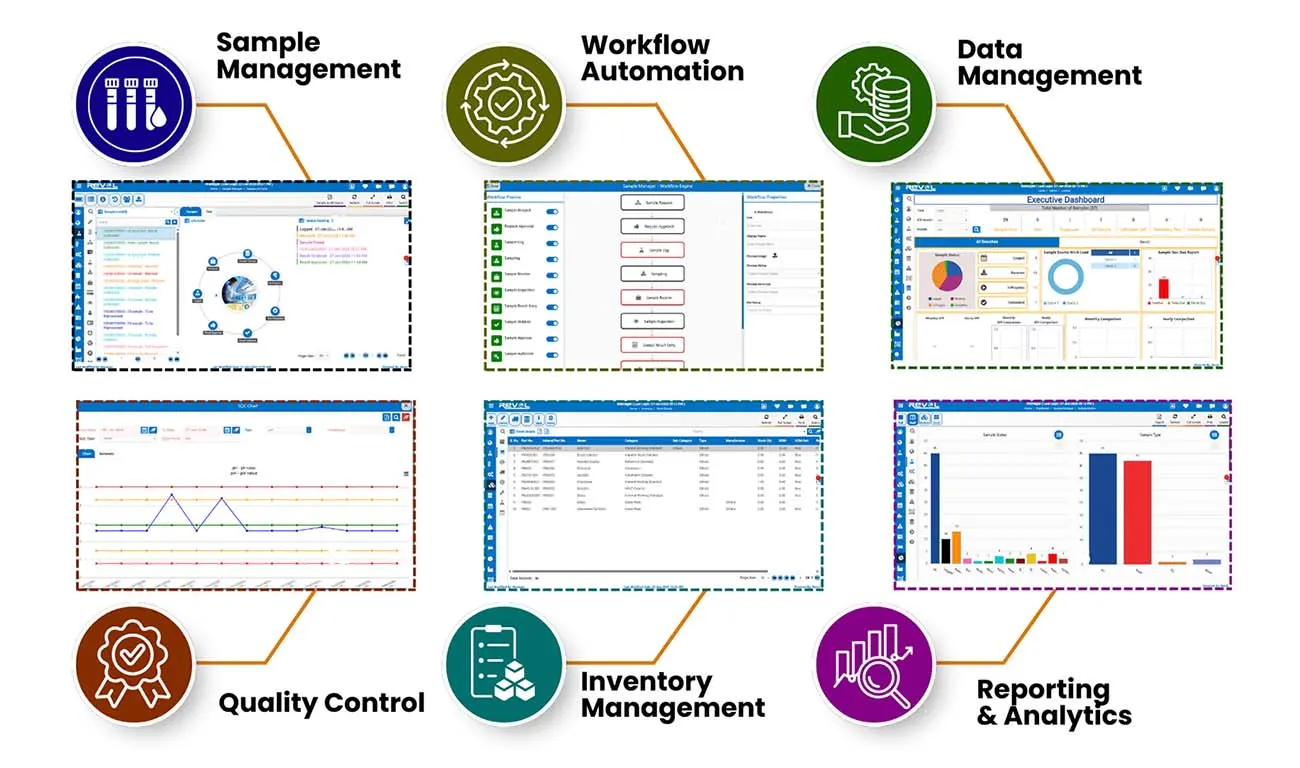
Complete Laboratory Management in One Platform
Sample Management
- Automated sample registration with barcode/RFID
- Real-time location tracking
- Chain of custody documentation
- Parent-child sample relationships
- Storage management with environmental monitoring
- Automated expiration alerts
Workflow Automation
- Configurable workflows for any testing process
- Automated test assignment and scheduling
- Batch processing capabilities
- Status tracking and notifications
- Electronic approval chains
- Mobile-friendly work queues
Data Management
- Instrument integration
- Multi-source data capture
- Automated calculations and validations
- Centralised data repository
- Advanced search and retrieval
- Data export in multiple formats
Quality Control
- Statistical process control (SQC/SPC)
- Control charting with automatic alerts
- Method validation tracking
- Proficiency testing management
- Non-conformance and CAPA workflows
- Deviation investigations
Inventory Management
- Reagent and consumable tracking
- Lot and batch management
- Expiration monitoring
- Standards and reference materials
- Equipment calibration scheduling
- Vendor management
Reporting & Analytics
- Configurable certificate templates
- Real-time KPI dashboards
- Trend analysis and visualisation
- Custom report builder
- Client portal access
- Advanced analytics with AI insights
LIMS by Industry
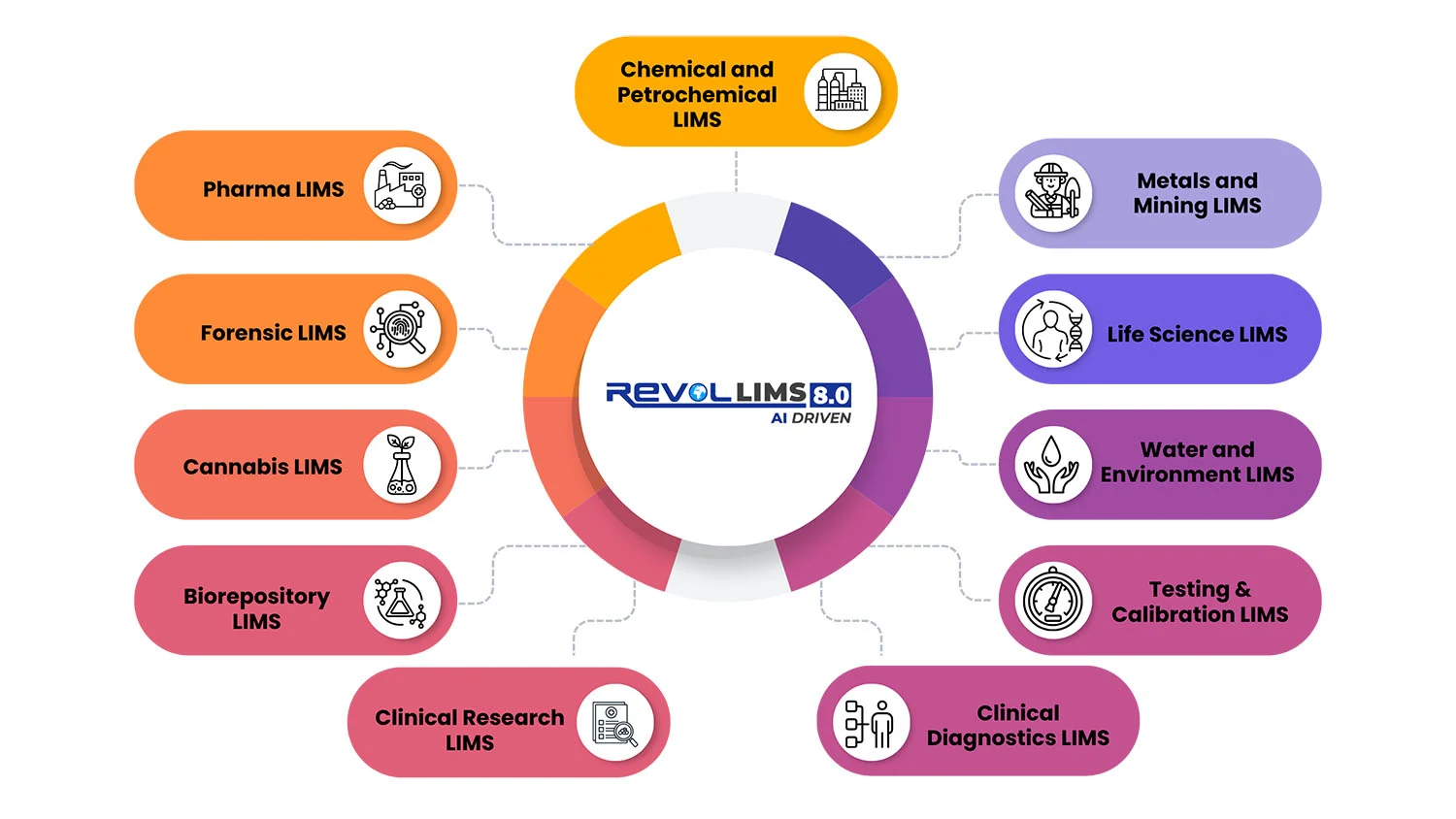
Specialised Solutions for Every Laboratory Type
Pharmaceutical & Biotechnology LIMS
- Stability study management
- Method validation and transfer
- Batch release testing
- Regulatory submission support
- Learn More →
Food Safety & Quality LIMS
- HACCP compliance
- Nutritional analysis
- Allergen testing workflows
- Supplier qualification
Environmental Testing LIMS
- EPA method compliance
- Field sampling workflows
- Water quality monitoring
- Soil and air testing
- Learn More →
Clinical Diagnostics LIMS
- Patient sample workflows
- CLIA compliance
- HL7 integration
- Result delivery to physicians
- Learn More →
Cannabis Testing LIMS
- State compliance
- Potency and contaminant testing
- Seed-to-sale integration
- COA generation
- Learn More →
- View All Industries →
LIMS Deployment Options
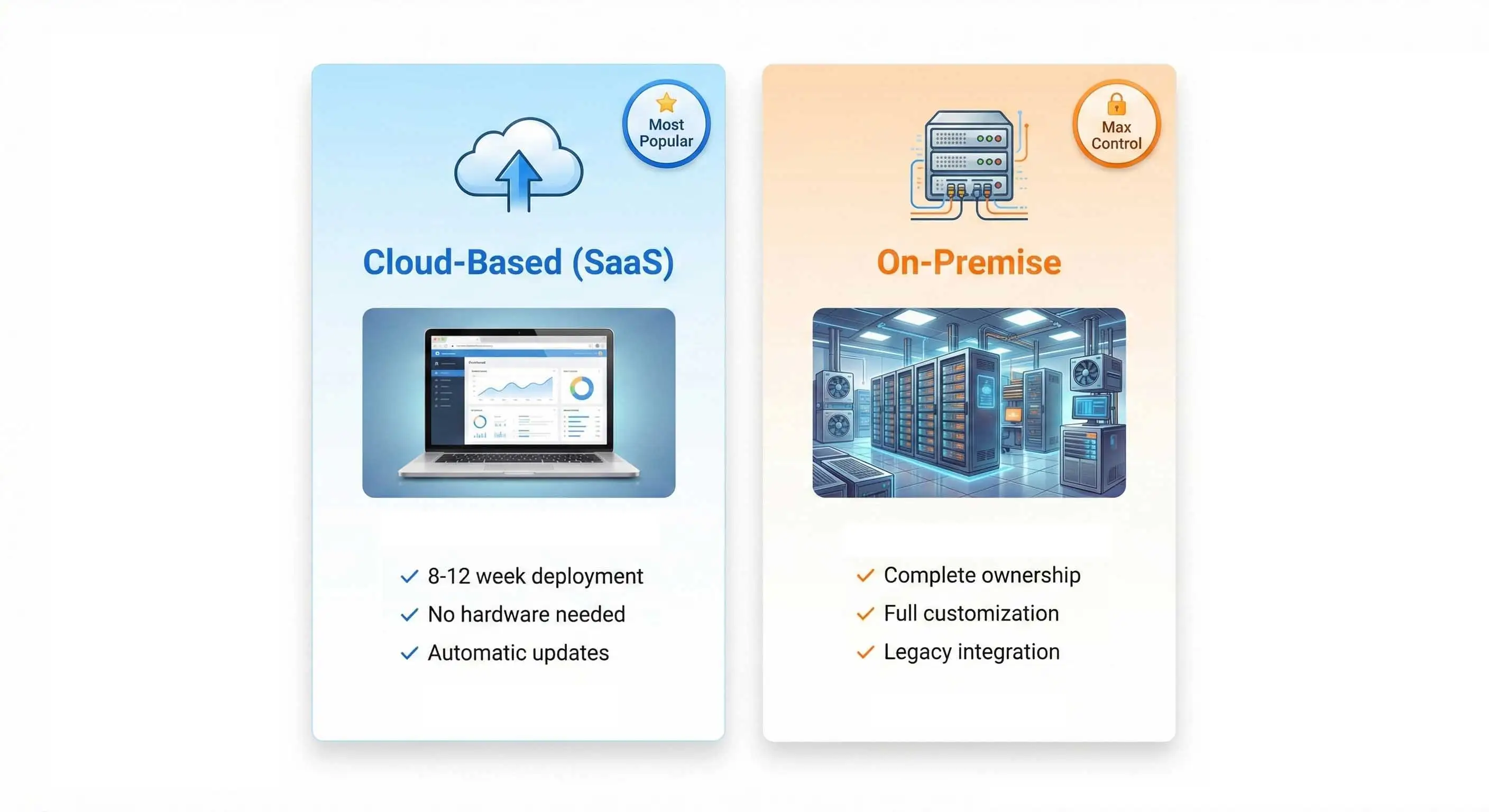
Choose the Best Fit for Your Laboratory
Cloud-Based LIMS (SaaS)
Fastest deployment, lowest upfront cost
- Go-live in 8-12 weeks
- No hardware infrastructure required
- Automatic updates and maintenance
- Predictable subscription pricing
- Access from anywhere
- Ideal for: Small to medium labs, multi-site operations
- View SaaS Pricing →
On-Premise LIMS
Maximum control and customization
- Complete data sovereignty
- Custom modifications
- Integration with legacy systems
- One-time licensing available
- Ideal for: Large enterprises, strict data requirements
- View On-Premise Pricing →
Why Revol LIMS Outperforms the Competition
| Feature | Revol LIMS | Legacy LIMS | Spreadsheets |
|---|---|---|---|
| Implementation Time | 12 weeks | 6–18 months | N/A |
| Total Cost of Ownership | $$$ | $$$$$ | N/A |
| AI Integration | ✓ Built-in | ✗ or Add-on | ✗ |
| Industry Templates | ✓ 15+ Industries | ✗ or Generic | ✗ |
| Compliance Ready | ✓ Out-of-box | Requires configuration | ✗ |
| Mobile Access | ✓ Native Apps | Limited | ✗ |
| Support Quality | 24/7 Dedicated | Limited hours | ✗ |
| User Training Required | Minimal | Extensive | N/A |
Some of our Success Stories
Laboratories That Transformed with Revol LIMS
Neoscience Labs - Commercial Testing
"Revol LIMS has become the backbone of our lab processes. We've been using it since 2017 and are extremely satisfied with the compliance features and support." — Moni Chelvan, Director
Key Results:
- ISO 17025 accreditation achieved
- 50% reduction in turnaround time
- Zero compliance violations
Arabian Gulf University - Molecular Medicine
"It has been more than 5 years filled with cooperation and satisfactory results on both operational and CAP accreditation levels." — Dr. Haytham Elhajjar, Business Development Director
ETLCO - Environmental Testing
"Revol LIMS is an outstanding tool that has become the backbone of our lab processes, making information highly organised and accessible." — Mohammad Saquib Khan, Lab Manager
View Case Studies →
View our G2 Reviews →
LIMS Implementation Process
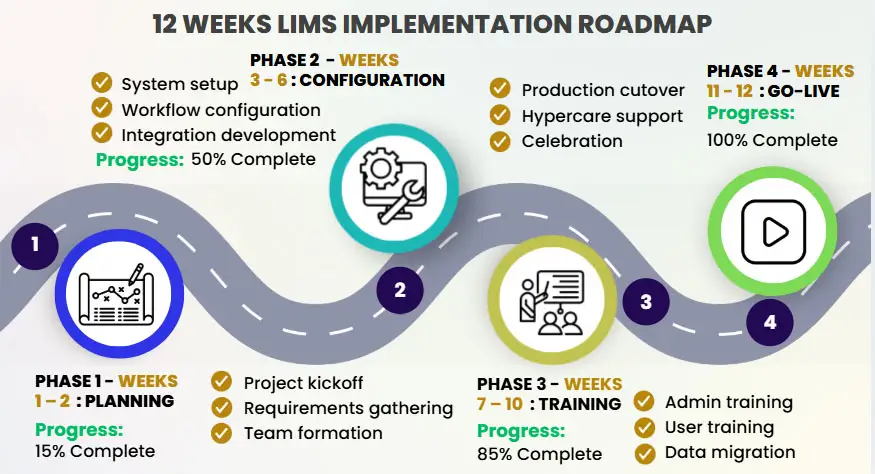
From Decision to Go-Live in 12 Weeks
Week 1-2: Planning & Requirements
- Project kick off and team formation
- Requirements gathering
- Workflow documentation
- Success criteria definition
Week 3-6: Configuration & Testing
- System configuration
- Workflow setup
- Integration development
- User acceptance testing
Week 7-10: Training & Migration
- Administrator training
- End-user training
- Data migration
- Validation documentation
Week 11-12: Go-Live & Support
- Cutover to production
- On-site support
- Hypercare period
- Performance optimisation
LIMS Features Checklist
Ensure Your LIMS Has What You Need
Essential Features:
- Sample tracking and chain of custody
- Configurable workflows
- Instrument integration
- Quality control and compliance
- Inventory management
- Reporting and analytics
- Electronic signatures
- Complete audit trails
Advanced Capabilities:
- AI-powered analytics
- Mobile application
- Client portal access
- Advanced statistics (SPC/SQC)
- Automated notifications
- Document management
- CAPA management
- API connectivity
Compliance Requirements:
- FDA 21 CFR Part 11
- ISO/IEC 17025
- GLP/GMP/GCP
- CLIA/CAP (clinical)
- HIPAA (healthcare)
- Data encryption
- Role-based security
- Backup and disaster recovery
Calculate Your Laboratory's Return on Investment
Average Benefits from Revol LIMS:
ROI Drivers:
1. Labor Cost Savings - Automation reduces manual work by 20-40%
2. Material Efficiency - Better inventory management cuts waste 10-20%
3. Reduced Errors - Eliminate rework and retesting costs by 50-80%
4. Faster Throughput - Process 30-50% more samples with existing staff
5. Compliance - Avoid regulatory penalties and warning letters
Frequently Asked Questions
How long does LIMS implementation take? Revol LIMS typically deploys in 12 weeks for standard implementations. Complex enterprise deployments may take 16-24 weeks. This is significantly faster than the industry average of 6-18 months.
What is the cost of LIMS? Pricing varies by deployment type and laboratory size. Cloud-based LIMS starts at $10,000-50,000 annually. On-premise solutions begin at $100,000+ for initial licenses. Revol LIMS offers 40% lower total cost of ownership than legacy platforms. View detailed pricing →
Can LIMS integrate with our instruments? Yes. Revol LIMS integrates with virtually all laboratory instruments, including HPLC, mass spectrometers, PCR machines, spectrophotometers, and more through direct interfaces, middleware, or file-based integration.
Is LIMS suitable for small laboratories? Absolutely. Cloud-based LIMS makes enterprise-grade laboratory management accessible to laboratories of all sizes with low upfront costs, rapid deployment, and minimal IT requirements.
Do we need dedicated IT staff? Cloud-based LIMS requires minimal IT support—the vendor handles infrastructure, security, and updates. On-premise LIMS requires dedicated IT resources for server management and maintenance.
How secure is our laboratory data? Revol LIMS employs multiple security layers: data encryption (in transit and at rest), role-based access controls, multi-factor authentication, complete audit trails, automated backups, and compliance with SOC 2, ISO 27001, and HIPAA standards.
What training is required? End users typically need 4-8 hours of initial training. System administrators require 2-5 days. Revol LIMS's intuitive interface minimises training requirements compared to legacy systems.
Can we try before buying? Yes. We offer live product demonstrations customised to your workflows, 14-30 day trial access, and proof-of-concept implementations to test with your actual data.
View All FAQs →
Getting Started with LIMS
Your Journey to Laboratory Excellence Begins Here
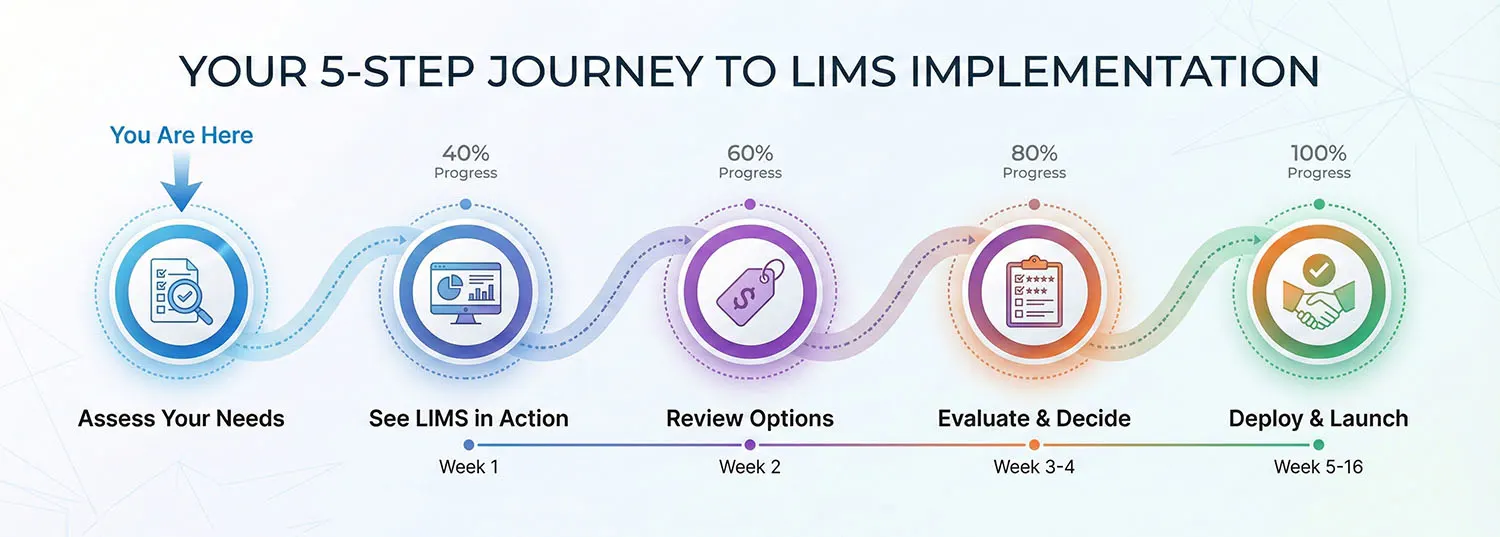
Step 1: Assess Your Needs Identify your laboratory's pain points, compliance requirements, and operational goals.
Step 2: See Revol LIMS in Action Schedule a personalized demonstration with workflows specific to your industry. Request Demo →
Step 3: Review Pricing Options Explore cloud, and on-premise deployment models.
Step 4: Evaluate & Decide Talk to reference customers, review case studies, and assess fit.
Step 5: Implementation & Go-Live Work with our expert team to deploy in 12 weeks.
Resources
Everything You Need to Know About LIMS
Educational Resources:
- Complete LIMS Guide - 15,000+ word comprehensive guide
- LIMS Key Features - Detailed feature breakdown
- LIMS Benefits - Business value analysis
- LIMS Modules - Functional capabilities
Industry Solutions:
Proof Points:
- Case Studies – Our success stories
- White Papers - Technical documentation
- Client Testimonials - Customer reviews
- Awards & Recognition
Technical Information:
Ready to Transform Your Laboratory?
Join laboratories worldwide that have modernised their operations with Revol LIMS.
Request Live Demo | View Pricing | Industries We Serve
Contact Information
Business Inquiries: marketing@revollims.com
Phone: +91 88071-12244
Office: 6/453 B, JS Tower, Hospital Road, Kanyakumari-629401, Tamil Nadu, India