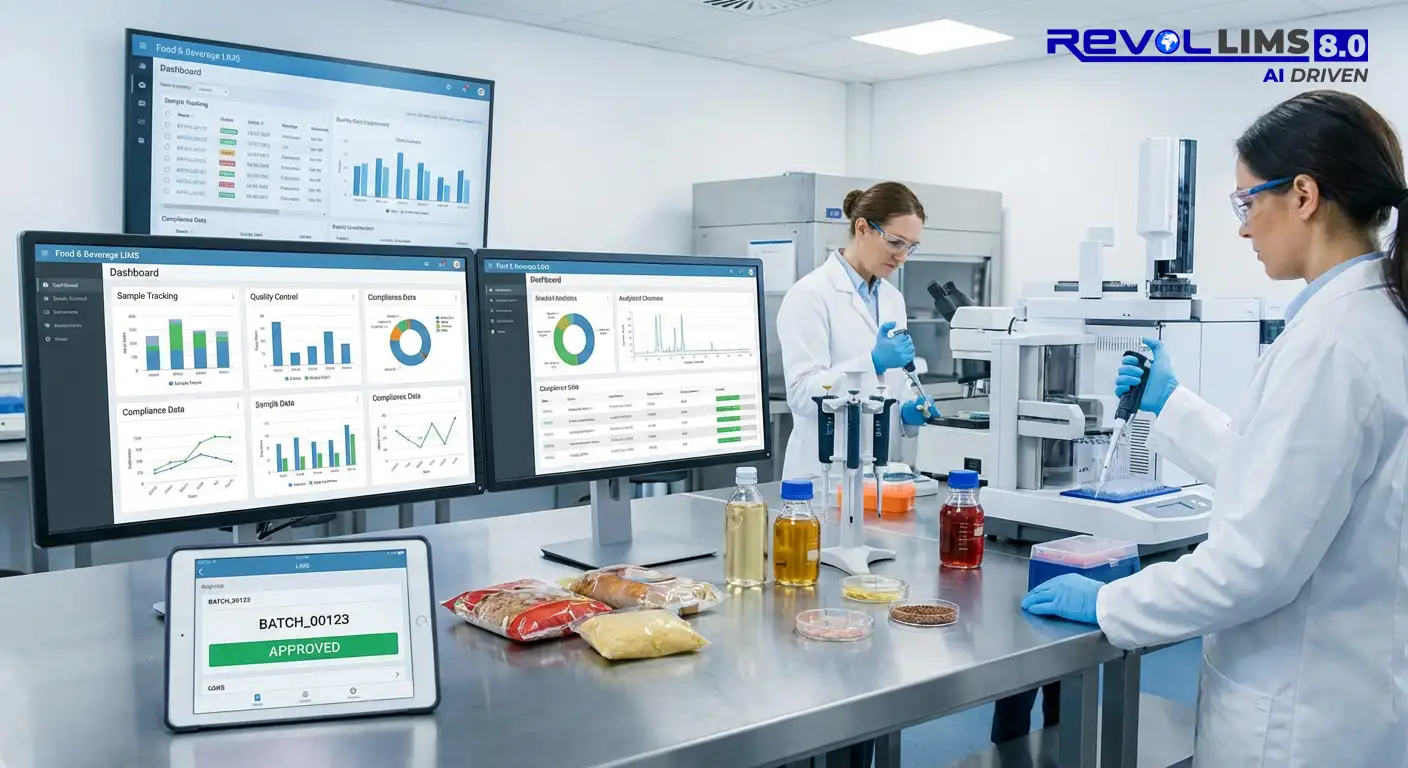
Enhances and Automates your Food and Beverage Laboratory Data Management and Quality
Revol Food and Beverage LIMS is a purpose-built solution that empowers laboratories in the food processing, beverage production, and food safety testing sectors to manage samples, enforce regulatory compliance, and automate quality workflows with precision and speed.
From raw material testing and shelf-life studies to allergen screening and microbiological analysis, Revol LIMS provides end-to-end visibility and control — ensuring your products reach consumers safely and in full compliance with global food safety standards.
Key Features of Revol Food and Beverage LIMS
Sample and Batch Management
- End-to-End Sample Tracking: Track every sample — from incoming raw materials to finished products — with real-time status updates, barcode or QR code labelling, and chain-of-custody logging to prevent mix-ups and ensure traceability.
- Batch and Lot Traceability: Link laboratory results directly to production batches and lot numbers, enabling rapid root-cause investigations and targeted recalls when deviations occur.
- Automated Sample Receipt and Login: Eliminate manual paperwork with automated sample login, pre-configured test assignment rules, and intelligent sample routing based on product type and priority.
Regulatory Compliance and Food Safety
- Global Food Safety Standards: Revol LIMS supports compliance with FDA 21 CFR Part 117 (FSMA), ISO 22000, HACCP, GFSI-recognized schemes (SQF, BRC, FSSC 22000), and regional food safety regulations, keeping your laboratory audit-ready at all times.
- Electronic Audit Trails: Every action — from sample login to result approval — is automatically logged with a timestamped, user-attributed audit trail that satisfies regulatory inspection requirements without manual recordkeeping.
- Automated Regulatory Reporting: Generate CoA (Certificate of Analysis), non-conformance reports, and regulatory submissions automatically, reducing turnaround time and eliminating transcription errors.
Data Integrity and Security
- Centralized Secure Data Repository: All laboratory data is stored in one tamper-evident, encrypted system — providing a single source of truth for QA managers, production teams, and auditors.
- Role-Based Access Control: Granular user permissions ensure that analysts, supervisors, and clients only access the data relevant to their role, maintaining confidentiality and data integrity.
- 21 CFR Part 11 Compliant Electronic Signatures: Enforce approval workflows with electronic signatures that meet FDA 21 CFR Part 11 requirements for electronic records in food safety contexts.
Quality Control and SQC/SPC
- Automated QC Checks and Alerts: Define acceptance criteria for microbiological, chemical, and physical parameters; LIMS automatically flags out-of-specification (OOS) results and triggers review workflows before product release.
- Statistical Quality Control (SQC/SPC): Monitor production trends with built-in control charts, Westgard rules, and capability indices to proactively detect process drift and prevent quality failures.
- CAPA Management Integration: Seamlessly link non-conformances to corrective and preventive action (CAPA) workflows, ensuring every deviation is tracked, resolved, and documented.
Workflow Automation
- Configurable Test Workflows: Pre-configure workflows for microbiological testing, nutritional analysis, allergen screening, heavy metals testing, and shelf-life studies — all ready to use without custom coding.
- Instrument Integration: Connect directly with HPLC, GC-MS, ICP-MS, PCR systems, and other analytical instruments for automatic result capture, eliminating transcription errors and accelerating turnaround.
- Automated Scheduling and Resource Management: Optimize analyst workloads and instrument utilisation with intelligent scheduling, reducing bottlenecks during high-volume testing periods.
Advanced Analytics and Reporting
- Real-Time Dashboards: Monitor lab throughput, pass/fail rates, and TAT (turnaround time) KPIs from a centralised dashboard — enabling laboratory managers to make data-driven decisions instantly.
- Customisable Certificate of Analysis (CoA): Generate professional, brand-consistent CoA documents automatically, with results mapped to customer specifications and regulatory limits.
- Trend Analysis and Predictive Insights: Leverage AI-powered analytics to identify quality trends, forecast shelf-life outcomes, and detect emerging contamination risks before they escalate.
Scalability, Cloud Access, and Deployment
- Flexible Deployment Options: Deploy Revol LIMS on-premise for maximum data control, in the cloud (SaaS/PaaS) for rapid go-live and remote access, or in a hybrid configuration — whichever suits your IT infrastructure.
- Multi-Site and Multi-Lab Support: Manage testing operations across multiple production facilities, contract labs, and regional offices from a single, unified LIMS instance.
- Mobile and Field Access: Enable field sampling teams and plant-floor QC personnel to log samples and access results via mobile devices, keeping data flowing in real time.
Compliance Requirements
- FDA 21 CFR Part 117 (FSMA – Preventive Controls for Human Food)
- FDA 21 CFR Part 11 (Electronic Records and Signatures)
- ISO 22000:2018 (Food Safety Management Systems)
- HACCP (Hazard Analysis and Critical Control Points)
- BRC Global Standard for Food Safety (Issue 9)
- SQF Food Safety Code
- FSSC 22000 (Food Safety System Certification)
- EU Regulation (EC) No 178/2002 (General Food Law)
- Codex Alimentarius Standards
- ISO/IEC 17025:2017 (Testing and Calibration Laboratories)
Key Functionalities
- Raw material, in-process, and finished product testing workflows
- Microbiological testing management (plate counts, PCR, pathogen detection)
- Allergen screening and cross-contamination tracking
- Nutritional analysis and labelling compliance
- Shelf-life and stability study management
- Automated Certificate of Analysis (CoA) generation
- Chain-of-custody and sample traceability
- Instrument integration with HPLC, GC-MS, ICP-MS, and PCR systems
- SQC/SPC charting with Westgard rules
- Non-conformance and CAPA management
- Customer and supplier portal for real-time result access
- Regulatory-ready audit trail and electronic signatures
- Environmental monitoring program management
- Multi-site and multi-language support
Get Started with Revol LIMS
Ready to transform your laboratory operations? Download our free brochure or request a personalised demo today. Our experts will walk you through how Revol LIMS can be tailored to your specific industry needs.
- Request Demo: https://revollims.com/lims-demo
- Download Brochure: https://revollims.com/lims-contact