Table Of Contents
- What is LIMS?
- What Does LIMS Stand For?
- The History and Evolution of LIMS
- How Does a LIMS Work?
- Core Functions and Features
- Key Benefits of Implementing LIMS
- Industries That Use LIMS
- LIMS vs LIS: Understanding the Difference
- Types of LIMS Solutions
- LIMS Implementation Guide
- How to Choose the Right LIMS Software
- Future Trends in LIMS Technology
- Frequently Asked Questions
What is LIMS?
A Laboratory Information Management System (LIMS) is specialised software that manages, tracks, and streamlines laboratory operations. LIMS serves as the digital backbone of modern laboratories, handling everything from sample registration and tracking to data analysis, quality control, and regulatory compliance.
Why LIMS Matters in 2026
In today's data-driven laboratory environment, LIMS has become essential infrastructure. Whether you're running a clinical diagnostics lab, pharmaceutical research facility, environmental testing center, or food safety laboratory, LIMS software eliminates manual processes, reduces errors by up to 90%, and ensures compliance with stringent regulatory requirements.
At its core, a LIMS system acts as a comprehensive database that organises and manages laboratory workflows, samples, instruments, standards, reagents, and analytical results. It provides real-time visibility into laboratory operations, enabling faster decision-making and improving operational efficiency by 30-50%.
The Modern Laboratory Challenge
Traditional laboratory operations relying on paper-based systems or spreadsheets face critical challenges that LIMS solves:
- Manual data entry errors: Transcription mistakes affect result accuracy
- Lost sample tracking: Difficulty locating samples wastes valuable time
- Slow report generation: Hours spent on manual documentation
- Compliance gaps: Incomplete audit trails risk regulatory violations
- Limited data accessibility: Information silos prevent collaboration
- Inefficient resource use: Poor inventory management increases costs
- No operational visibility: Managers lack real-time performance insights
LIMS software addresses these challenges by automating workflows, centralising data management, and providing powerful analytical capabilities that transform laboratory operations.
What Does LIMS Stand For?
LIMS stands for Laboratory Information Management System.
The acronym LIMS is universally recognised across the laboratory and scientific community. Some organisations may also refer to similar systems as:
- Laboratory Information System (LIS) - typically for clinical laboratory systems
- Lab Management Software - a generic term for laboratory software
- Laboratory Management System - broader operational management
- Laboratory Data Management System - focused on data handling
While these terms are sometimes used interchangeably, LIMS specifically refers to comprehensive laboratory management software that handles the full spectrum of operations, from sample intake to final reporting and data archival.
The History and Evolution of LIMS
The Early Days (1980s)
The concept of LIMS emerged in the early 1980s when laboratories recognised the need for computerised sample tracking and data management. First-generation LIMS were custom-built mainframe systems developed by large pharmaceutical companies and government laboratories to manage growing volumes of samples and data.
These early systems were expensive, required significant IT infrastructure, and focused primarily on basic sample tracking and data storage. Only large organisations with substantial budgets could afford LIMS technology.
Commercial LIMS Era (1990s-2000s)
The 1990s saw the emergence of commercial off-the-shelf LIMS solutions running on client-server architectures. This period democratized LIMS technology, making it accessible to medium-sized laboratories. Key developments included:
- Windows-based user interfaces replacing command-line systems
- Standardised database technologies like Oracle and SQL Server
- Integration capabilities with laboratory instruments
- Industry-specific LIMS solutions for clinical, pharmaceutical, and environmental testing
- Compliance features for FDA 21 CFR Part 11 and ISO standards
Modern Cloud-Based LIMS (2010s-Present)
The past decade has witnessed a transformation in LIMS technology driven by cloud computing, mobile technology, and advanced analytics:
- Cloud-based SaaS LIMS eliminates on-premise infrastructure needs
- Mobile accessibility enabling laboratory management from anywhere
- Advanced integration with ERP, CRM, and enterprise systems
- Artificial Intelligence and Machine Learning for predictive analytics
- IoT integration for real-time instrument monitoring
- Blockchain technology for enhanced data integrity
- Open architecture with API-first design for seamless connectivity
Today's LIMS solutions are more flexible, user-friendly, and powerful than ever before. The global LIMS market reached $2.49 billion in 2024 and is projected to grow at a 9.8% CAGR, reaching $5.26 billion by 2032, reflecting increasing adoption across industries worldwide.
How Does a LIMS Work?
Understanding the LIMS workflow is essential to appreciating how this software transforms laboratory operations. Here's a detailed look at how LIMS manages the complete laboratory sample lifecycle.
Step 1: Sample Registration and Accessioning
The LIMS journey begins when a sample enters the laboratory. Samples are registered into the system either manually by laboratory staff or automatically through barcode scanning, RFID tags, or integration with client portals. Each sample receives a unique identifier that follows it throughout its lifecycle, and critical metadata is captured, including sample type, collection date and time, source location, requested tests, priority level, and client information. The system establishes a complete chain of custody, documenting who handled the sample and when.
Step 2: Sample Tracking and Location Management
Once registered, LIMS provides real-time visibility into sample status and location. The system tracks exactly where each sample is at any given moment, whether in receiving, storage, testing area, or disposal. LIMS manages sample storage conditions, expiration dates, and retrieval protocols. Staff scan samples using barcodes or RFID as they move between locations, automatically updating the system. Visual dashboards allow laboratory managers to view sample status, identify bottlenecks, and monitor throughput in real-time.
Step 3: Test Assignment and Workflow Management
LIMS intelligently manages which tests need to be performed and by whom. Based on sample type and client requests, the system automatically assigns appropriate tests and schedules them based on priority, resource availability, and dependencies between tests. Analysts receive prioritised work lists showing which samples to process, and the system optimally allocates instruments, reagents, and personnel.
Step 4: Test Execution and Data Collection
During testing, LIMS captures data and monitors quality. The system directly interfaces with analytical instruments like HPLC, mass spectrometers, and PCR machines to automatically capture results. For instruments without integration, technicians enter results directly into LIMS with built-in validation rules. The system monitors test progress and alerts staff to any issues or delays, while electronic signatures document who performed each step and when.
Step 5: Quality Control and Data Validation
Quality assurance is integrated throughout the LIMS workflow. The system automatically schedules and tracks quality control samples, including blanks, duplicates, and standards. LIMS performs complex calculations, conversions, and statistical analyses. Data is automatically checked against specification limits, control charts, and validation criteria. The system flags results that fall outside acceptable ranges for immediate review, and results follow defined approval chains before being released.
Step 6: Report Generation and Delivery
LIMS automates the creation and distribution of test reports. Professional reports are generated automatically using configurable templates and can be produced in PDF, Excel, Word, or other formats. Reports are automatically sent to clients via email, portal access, or system-to-system integration. Reports can be customised to meet specific client or regulatory requirements, and formal certificates of analysis are generated with all required quality and compliance information.
Step 7: Data Archival and Audit Trail
Finally, LIMS ensures long-term data integrity and compliance. All data, including raw instrument files, is securely stored according to regulatory requirements. Every action in the system is logged with user ID, timestamp, and details of what changed. The system automatically manages data retention schedules based on regulatory requirements. Historical data can be quickly searched and retrieved for trend analysis, investigations, or audits. Automated backup ensures data is never lost.
Continuous Improvement Loop
Modern LIMS also enable continuous improvement through management dashboards showing key performance indicators like turnaround time, throughput, and error rates. Historical data reveals patterns and opportunities for optimisation. The system tracks instrument usage, staff productivity, and inventory consumption, while capturing and analysing customer satisfaction data.
This comprehensive workflow demonstrates how LIMS transforms a complex, error-prone manual process into a streamlined, automated, and fully traceable digital workflow that enhances efficiency, quality, and compliance.
Core Functions and Features of LIMS Software
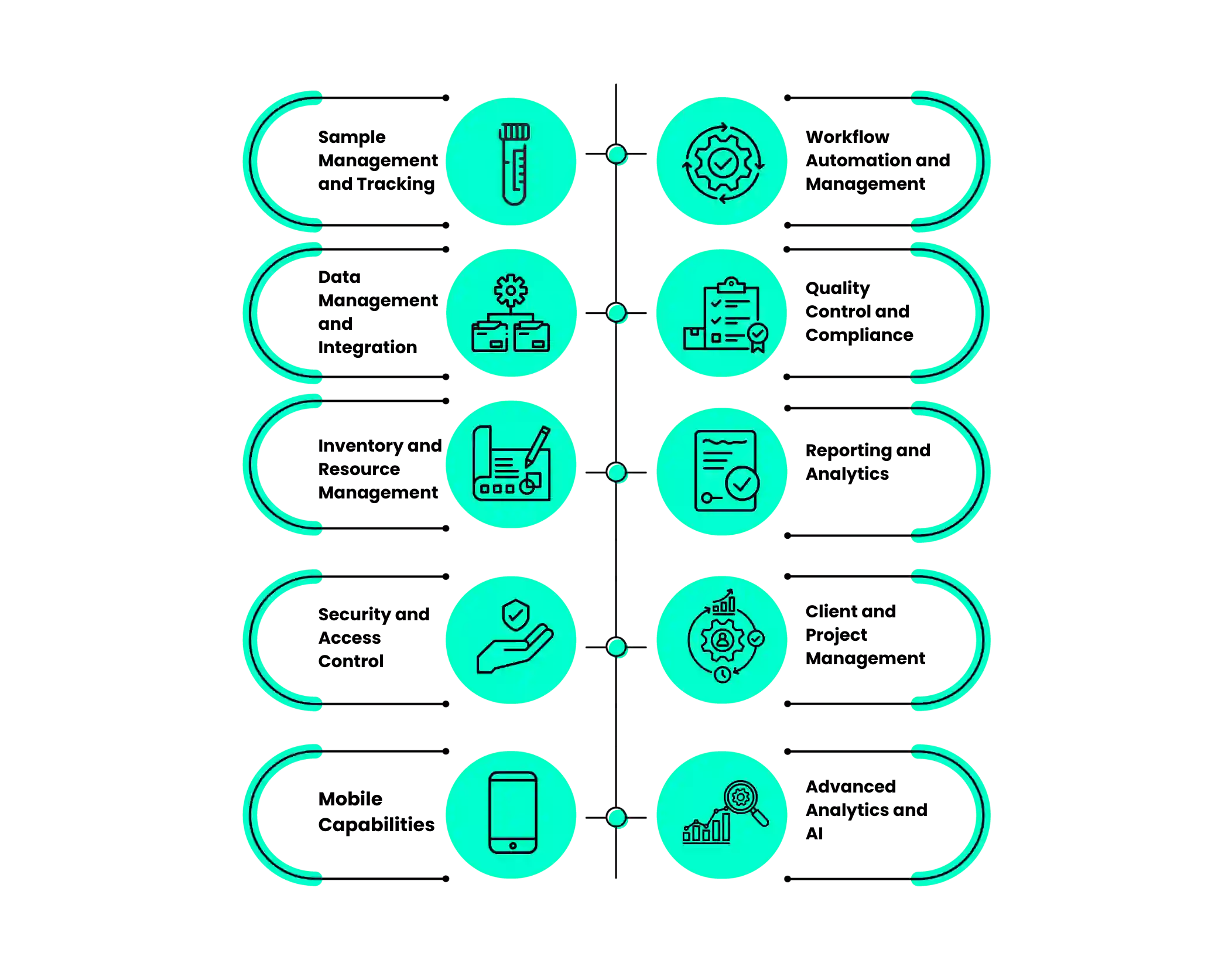
Modern LIMS platforms offer comprehensive capabilities designed to address every aspect of laboratory management.
1. Sample Management and Tracking
LIMS provides complete sample lifecycle management, including sample registration with unique identification using barcodes, QR codes, or RFID. The system documents and tracks the chain of custody, provides real-time sample location tracking throughout the laboratory, and tracks parent-child relationships between samples. Storage management includes location assignment, temperature monitoring, and retrieval. The system handles sample pooling and aliquoting for dividing samples into multiple portions and provides automatic expiration management with alerts for samples requiring disposal.
2. Workflow Automation and Management
LIMS offers configurable workflows tailored to specific laboratory processes. The system provides automated test assignment based on sample type and client requirements, work queue management with prioritisation and load balancing, and batch processing for grouping similar samples for efficiency. Status tracking and notifications keep stakeholders informed of progress. The system includes scheduling and resource planning for optimal resource utilisation and workflow visualization showing sample progress through testing stages.
3. Data Management and Integration
LIMS serves as a centralised data repository for all laboratory information. The system provides instrument integration with bi-directional communication, multi-source data capture from instruments, manual entry, and imported files. Data validation and verification use configurable business rules. The system performs calculations and formulas for automated result computation, handles units of measure conversion and standardisation, and provides data import/export capabilities in multiple formats. API connectivity enables integration with ERP, ELN, SDMS, and other enterprise systems.
4. Quality Control and Compliance
LIMS manages quality control samples, including blanks, standards, duplicates, and spikes. The system provides control charting with automatic out-of-control detection using Westgard rules and Levey-Jennings charts. Specification management provides automatic pass/fail determination. The system tracks method validation and performance verification, manages proficiency testing and trending, and handles non-conformance tracking with corrective action workflows. Deviation management includes investigation and resolution tracking. Compliance features support FDA 21 CFR Part 11, ISO 17025, GLP, GMP, and other regulations.
5. Inventory and Resource Management
LIMS tracks reagents and consumables with lot and batch management. The system provides expiration management with automated alerts, tracks standards and reference materials with certification management, and schedules equipment maintenance with service history. Calibration management includes automatic reminder notifications. The system handles inventory reordering with configurable minimum stock levels, tracks costs by sample, client, or project, and manages vendor information for procurement tracking.
6. Reporting and Analytics
LIMS provides configurable report templates for certificates of analysis, batch reports, and summary reports. Custom dashboards show real-time KPIs and metrics. The system offers trend analysis and visualisation with charts and graphs, ad-hoc query tools for flexible data exploration, and statistical analysis, including capability studies and control charting. Management reports cover turnaround time, productivity, and quality metrics. Client-specific reporting uses customizable formats and branding. The system exports to external tools like Excel, Tableau, or Power BI.
7. Security and Access Control
LIMS implements role-based access control, restricting functionality by user role. The system provides electronic signatures compliant with regulatory requirements and maintains a complete audit trail, logging all system activities. Data encryption protects information both in transit and at rest. User authentication includes multi-factor authentication options. Password management enforces complexity requirements and expiration. The system implements data privacy controls for protecting sensitive information and provides secure data backup and disaster recovery capabilities.
8. Client and Project Management
LIMS offers client portals for sample submission and result access. The system provides project tracking with hierarchical organisation, contract management with pricing and service level agreements, and quote generation with approval workflows. Invoicing integration connects with billing systems. Client communication tools include automated notifications. The system includes customer relationship management features and service level tracking with compliance monitoring.
9. Mobile Capabilities
LIMS provides mobile apps for iOS and Android devices. The system supports field sampling with GPS location capture, mobile result review and approval, and barcode scanning using mobile device cameras. Offline data capture provides automatic synchronisation. Push notifications alert users to urgent actions. The system offers mobile dashboard access for remote monitoring.
10. Advanced Analytics and AI
Modern LIMS incorporates predictive maintenance using machine learning algorithms. The system provides anomaly detection for identifying unusual patterns and automated data review, flagging potential issues. Intelligent sample routing optimises workflow efficiency.
Result prediction uses historical data. Natural language query simplifies data access. Recommendation engines support method optimisation.
These comprehensive features work together to create a powerful, integrated laboratory management ecosystem that drives efficiency, ensures quality, and maintains regulatory compliance.
Key Benefits of Implementing LIMS
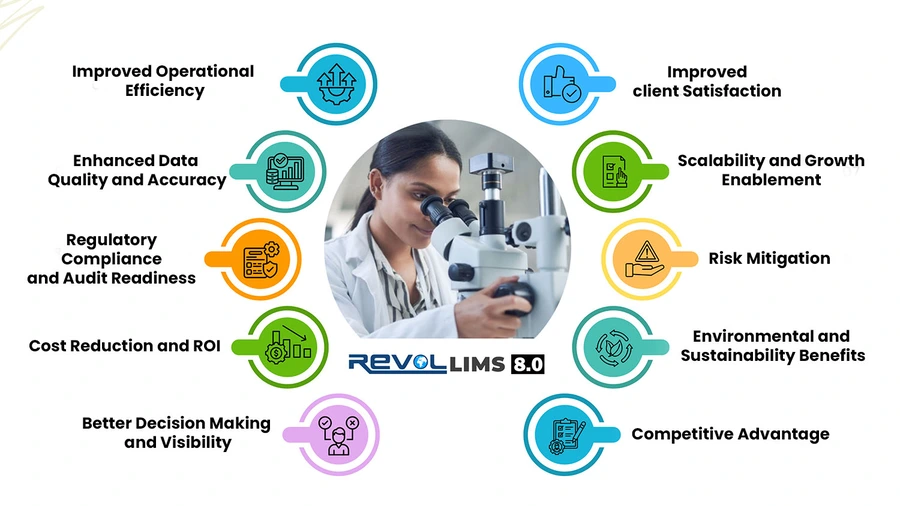
Implementing a Laboratory Information Management System delivers transformative benefits across every aspect of laboratory operations. Organisations that deploy LIMS typically see rapid returns on investment through multiple value drivers.
1. Improved Operational Efficiency
LIMS reduces sample turnaround time by 30-50% through workflow automation. The system eliminates hours spent on manual data entry and transcription, accelerates report generation from hours to minutes, and streamlines sample tracking. Process optimisation automates repetitive tasks, freeing staff for higher-value activities. The system eliminates bottlenecks through real-time visibility and load balancing, optimises resource utilisation across instruments, personnel, and materials, and enables parallel processing of samples for increased throughput. Productivity gains include increasing laboratory throughput by 20-40% without adding staff, processing more samples with existing resources, and reducing administrative burden on laboratory personnel.
2. Enhanced Data Quality and Accuracy
LIMS eliminates over 90% of transcription errors through automated data capture. The system reduces calculation errors through automated formulas and validations, prevents sample mix-ups with positive identification systems, and minimises lost samples through comprehensive tracking. Data integrity ensures complete, accurate, and consistent data across all operations. The system implements validation rules that catch errors before they propagate and maintains data integrity through version control and change management. Quality improvements include standardising procedures across all laboratory personnel, ensuring consistent application of quality control procedures, detecting out-of-specification results immediately, and tracking quality metrics for continuous improvement.
3. Regulatory Compliance and Audit Readiness
LIMS meets FDA 21 CFR Part 11 requirements for electronic records and signatures, achieves ISO/IEC 17025 compliance for testing and calibration laboratories, and supports GLP, GMP, and GCP requirements in regulated industries. The system maintains CLIA compliance for clinical laboratories. Complete audit trails automatically document every action with user, date, time, and details. The system creates a comprehensive chain of custody records, tracks all changes to data with before and after values, and generates audit reports in minutes instead of days. For inspections, LIMS enables laboratories to respond to regulatory inspections with confidence, quickly retrieve historical data and documentation, demonstrate procedural consistency and quality controls, and reduce inspection preparation time by 70-80%.
4. Cost Reduction and ROI
Direct cost savings include reducing labour costs through automation and efficiency gains, minimising material waste through better inventory management, decreasing rework and retesting costs through improved accuracy, and lowering IT infrastructure costs with cloud-based solutions. Indirect savings include avoiding regulatory penalties and warning letters, reducing insurance costs through documented quality systems, minimising product recalls through better traceability, and decreasing legal exposure through comprehensive documentation. Revenue enhancement enables increasing testing capacity for revenue growth, improving client satisfaction, leading to repeat business, enabling new service offerings through enhanced capabilities, and reducing turnaround time, attracting premium-paying clients.
Organisations typically achieve positive ROI within 6-18 months, with many seeing returns of 200-400% over five years.
5. Better Decision Making and Visibility
LIMS provides real-time insights through access to current operational status via live dashboards. The system identifies bottlenecks and issues as they occur, monitors key performance indicators continuously, and enables data-driven decisions based on accurate information. Strategic analytics include analysing trends across time periods, clients, or sample types, identifying opportunities for process improvement, forecasting resource requirements based on historical patterns, and benchmarking performance against industry standards. Management reporting provides executive dashboards showing critical business metrics, tracks profitability by client, project, or service line, monitors quality metrics and trends, and demonstrates value to stakeholders with concrete data.
6. Improved Client Satisfaction
Service quality delivers faster turnaround times exceeding client expectations, provides accurate and reliable results consistently, offers professional branded reports and certificates, and enables real-time status updates, reducing client inquiries. Client portals allow 24/7 access to results and historical data, enable electronic sample submission, reducing paperwork, provide transparency into testing progress, and facilitate data export in client-preferred formats. Enhanced communication automates notifications at key milestones, reduces phone calls and emails through self-service options, enables faster responses to client inquiries with instant data access, and builds trust through demonstrated quality systems.
7. Scalability and Growth Enablement
LIMS supports business growth by scaling operations without proportional increases in staff, adding new test types and services rapidly, expanding to multiple locations with centralised management, and supporting mergers and acquisitions through system consolidation. Flexibility enables adapting quickly to changing business requirements, configuring workflows for new service offerings, integrating new instruments and technologies easily, and customising to meet client-specific requirements. Future-proofing leverages cloud-based architecture for continuous updates, adopts new technologies like AI and IoT as they emerge, integrates with evolving enterprise systems, and prepares for future regulatory requirements.
8. Risk Mitigation
Sample integrity prevents sample loss through comprehensive tracking, ensures proper storage conditions with automated monitoring, maintains chain of custody for legal defensibility, and detects sample handling issues quickly. Business continuity protects data through automated backup and disaster recovery, maintains operations during emergencies with cloud access, reduces dependency on individual staff members through standardisation, and ensures institutional knowledge is preserved in the system. Compliance risk avoids regulatory warning letters and penalties, prevents data integrity issues that could invalidate studies, ensures consistent application of procedures, and maintains defensible documentation for legal proceedings.
9. Environmental and Sustainability Benefits
Resource conservation reduces paper consumption through electronic records and reports, optimises reagent and consumable usage through better planning, minimises waste through improved inventory management, and decreases energy consumption through efficient scheduling. Sustainability reporting tracks environmental metrics and compliance, monitors waste generation and disposal, documents sustainability initiatives, and reports on environmental goals and achievements.
10. Competitive Advantage
Market differentiation demonstrates superior quality management to prospective clients, obtains competitive certifications and accreditations, offers faster turnaround times than competitors, and provides superior client experience through technology. Innovation enables adopting new testing methods and technologies faster, participating in research and development more effectively, attracting top talent seeking modern work environments, and positioning as an industry leader in quality and technology.
These comprehensive benefits demonstrate why LIMS has become essential infrastructure for modern laboratories. The combination of operational efficiency, quality improvement, compliance assurance, and client satisfaction creates a compelling value proposition that delivers measurable returns across all dimensions of laboratory performance.
Industries That Use LIMS
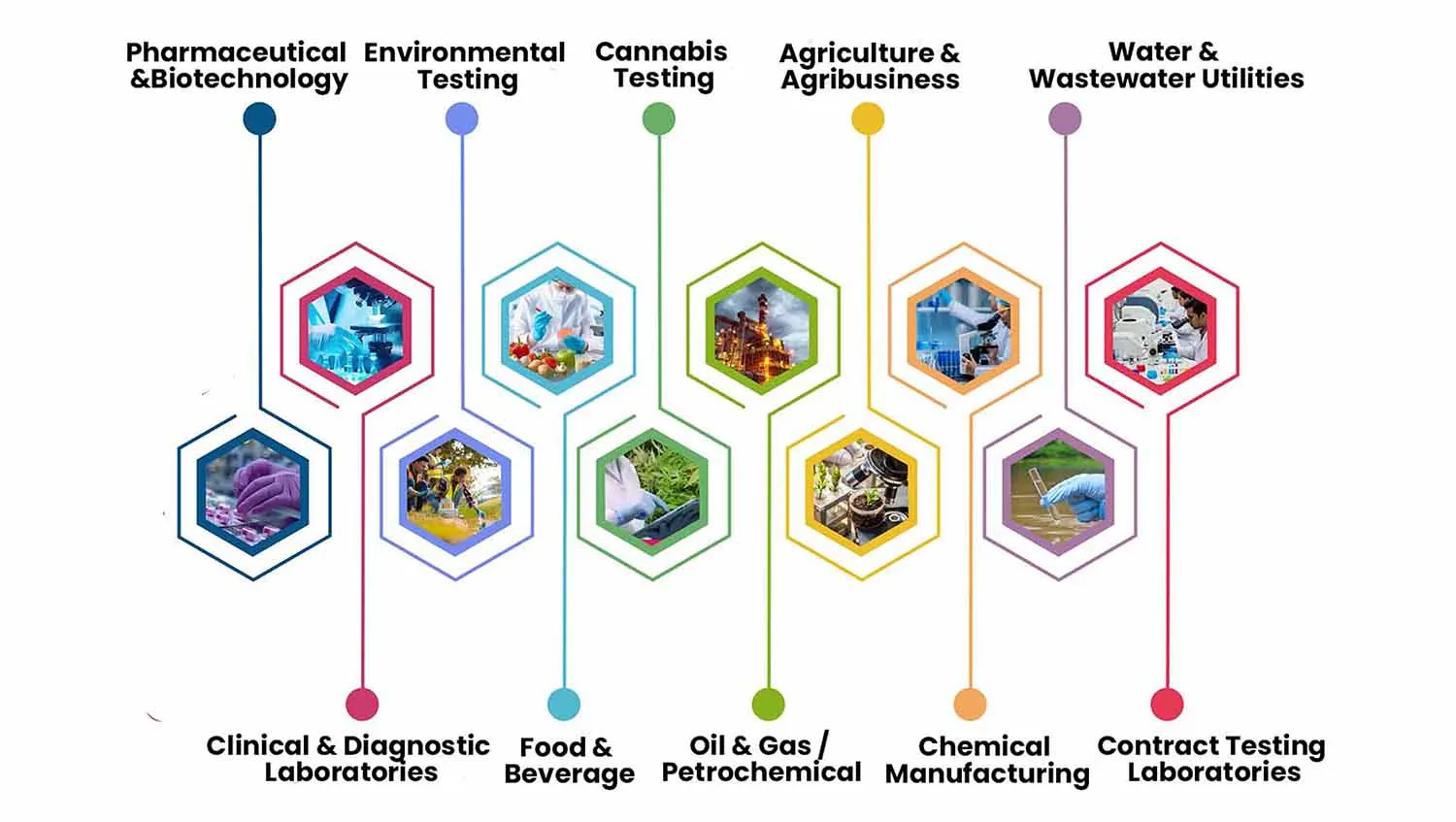
1. Pharmaceutical and Biotechnology
Pharmaceutical and biotech companies use LIMS for drug development and clinical trials management, quality control and release testing for finished products, raw material and excipient testing, stability studies and shelf-life determination, method validation and transfer, and regulatory submission support. Key requirements include 21 CFR Part 11 compliance for electronic records, GMP and GLP regulatory compliance, integration with ELN and SDMS systems, and support for complex workflows and multi-site operations.
2. Clinical and Diagnostic Laboratories
Clinical laboratories use LIMS for patient sample management from collection to reporting, clinical chemistry, haematology, microbiology, and molecular diagnostics, anatomical pathology and histology, blood banking and transfusion services, point-of-care testing coordination, and result delivery to physicians and health information systems. Key requirements include CLIA compliance for clinical laboratories, HL7 integration with hospital information systems, HIPAA compliance for patient data privacy, and rapid turnaround time tracking and management.
3. Environmental Testing
Environmental testing laboratories use LIMS for water quality testing for drinking water, wastewater, and surface water, soil and groundwater contamination analysis, air quality monitoring and emissions testing, environmental impact assessments, hazardous waste characterisation, and compliance monitoring for environmental regulations. Key requirements include EPA method compliance and documentation, field sampling data capture, geographic information system integration, and multi-client project management.
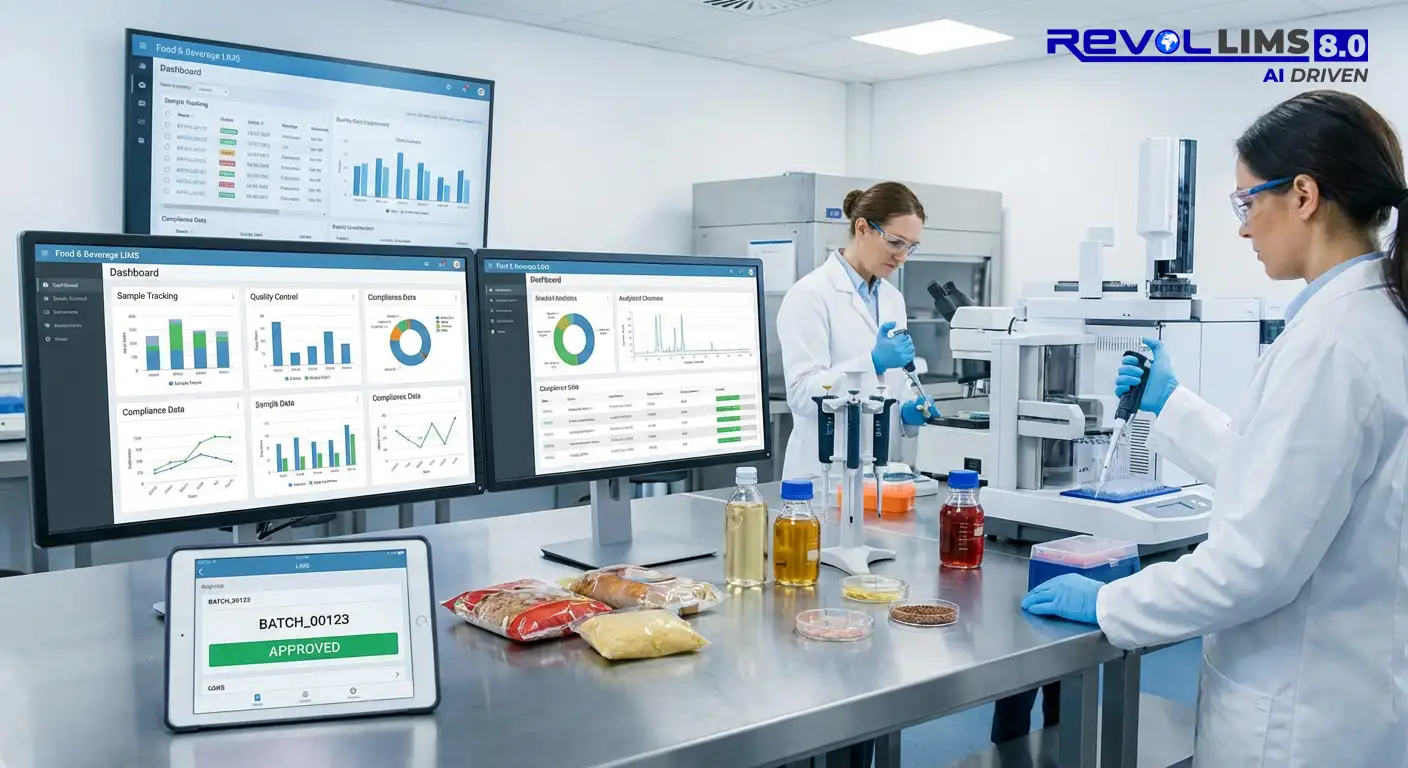
4. Food and Beverage
Food and beverage companies use LIMS for food safety testing, including pathogens, allergens, and contaminants, nutritional analysis and labelling verification, quality control during production, shelf-life and stability studies, supplier qualification testing, and organic and GMO certification testing. Key requirements include HACCP and food safety plan support, traceability from farm to fork, rapid pathogen detection workflows, and certificate of analysis generation.
5. Cannabis Testing
Cannabis testing laboratories use LIMS for potency testing to determine cannabinoid profiles, pesticide and heavy metal screening, microbial contamination testing, residual solvent analysis, terpene profiling, and compliance testing for regulatory approval. Key requirements include state-specific regulatory compliance, seed-to-sale tracking integration, rapid turnaround for perishable products, and secure chain of custody documentation.
6. Oil and Gas / Petrochemical
Oil and gas companies use LIMS for crude oil assay and characterisation, fuel quality testing, lubricant analysis, pipeline integrity testing, environmental monitoring at facilities, and quality control for refinery operations. Key requirements include ASTM method compliance, high-volume sample processing, integration with process control systems, and multi-location management.
7. Agriculture and Agribusiness
Agricultural laboratories use LIMS for soil fertility and nutrient analysis, plant tissue analysis, seed testing and certification, pesticide residue monitoring, GMO detection, and feed and fertiliser analysis. Key requirements include field sampling workflows, geographic tracking of samples, agronomic recommendation generation, and mobile data collection.
8. Chemical Manufacturing
Chemical manufacturers use LIMS for raw material qualification, in-process quality control, finished product release testing, method development and validation, customer complaint investigation, and regulatory compliance testing. Key requirements include ERP integration for production scheduling, specification management, global harmonisation across facilities, and batch genealogy tracking.
9. Water and Wastewater Utilities
Water and wastewater utilities use LIMS for drinking water quality monitoring, wastewater treatment monitoring, regulatory compliance testing, distribution system monitoring, process optimisation, and consumer complaint investigation. Key requirements include EPA Safe Drinking Water Act compliance, NPDES permit monitoring, SCADA system integration, and public health reporting.
10. Contract Testing Laboratories
Contract testing laboratories use LIMS for multi-industry testing services, method development and validation, regulatory consulting and support, specialised analytical services, research and development support, and expert witness and litigation support. Key requirements include multi-client management, flexible workflow configuration, complex pricing and invoicing, and project-based organisation.
Additional Industries
LIMS is also widely used in academic and research institutions for research project management, teaching laboratory coordination, core facility operations, multi-investigator data sharing, grant-funded project tracking, and publication and data archival. Other sectors include cosmetics and personal care, mining and metals, forensic laboratories, and veterinary diagnostics.
The versatility of modern LIMS solutions allows them to be configured for virtually any laboratory testing environment, making them truly universal tools for laboratory management across industries.
LIMS vs LIS: Understanding the Difference
One of the most common questions in laboratory management is understanding the distinction between LIMS and LIS. While the terms are sometimes used interchangeably, there are important differences.
What is LIS?
A Laboratory Information System (LIS) is specialised software primarily designed for clinical laboratories that perform diagnostic testing on patient samples. LIS solutions are optimised for healthcare environments and integrate closely with hospital information systems, electronic medical records, and other healthcare IT infrastructure.
Key Differences: LIMS vs LIS
Primary Focus:
LIMS serves research, industrial, and environmental laboratories, while LIS focuses on clinical and diagnostic laboratories.
Sample Types:
LIMS handles varied samples, including water, soil, chemicals, food, and pharmaceuticals, while LIS primarily manages patient samples such as blood, urine, and tissue.
Integration:
LIMS integrates with ERP, CRM, and manufacturing systems, while LIS connects with hospital information systems, EMR, billing systems, and insurance systems.
Regulatory Focus:
LIMS complies with EPA, FDA, ISO 17025, GLP, and GMP regulations, while LIS meets CLIA, CAP, HIPAA, and HL7 standards.
Workflow:
LIMS offers highly configurable workflows for diverse testing types, while LIS provides standardised workflows for clinical diagnostic procedures.
Reporting:
LIMS generates certificates of analysis and compliance reports, while LIS produces patient test results and diagnostic reports.
Patient Data:
LIMS is generally not patient-focused, while LIS makes patient demographics and medical records central to operations.
Billing:
LIMS uses project-based client invoicing, while LIS employs medical coding, insurance claims, and CPT codes.
Turnaround Time:
LIMS handles variable timeframes from hours to weeks, while LIS often manages urgent cases requiring minutes to hours.
Users:
LIMS serves research scientists, QC analysts, and lab technicians, while LIS supports medical technologists, pathologists, and physicians.
When to Choose LIMS vs LIS
Choose LIMS if you are a pharmaceutical, biotech, or research laboratory, an environmental, food safety, or agricultural testing lab, a contract testing laboratory serving multiple industries, a manufacturing quality control laboratory, a research institution or university laboratory, or any non-clinical laboratory requiring flexible workflows.
Choose LIS if you are a hospital or medical center laboratory, an independent diagnostic testing facility, a physician office laboratory, a reference clinical laboratory, a blood bank or transfusion service, or any clinical laboratory requiring CLIA compliance.
The Convergence: Modern Unified Solutions
The line between LIMS and LIS has become increasingly blurred in recent years. Many modern laboratory software solutions offer capabilities that span both domains. Unified laboratory systems provide core LIMS functionality for sample management and workflow, clinical laboratory features for patient testing, a single platform reducing integration complexity, consistent user experience across laboratory types, and shared data infrastructure.
Some laboratories, particularly large hospital systems with both clinical and research operations, implement both systems with LIS for patient testing and diagnostic workflows, LIMS for research, clinical trials, and specialised testing, and integration between the two systems for seamless operations.
Making the Right Choice
When selecting laboratory software, consider your primary laboratory function, regulatory requirements, integration needs, workflow complexity, budget and resources, and future needs. For most research, industrial, environmental, food safety, pharmaceutical, and quality control laboratories, LIMS is the appropriate choice. For clinical diagnostic laboratories performing patient testing, LIS is typically the better fit.
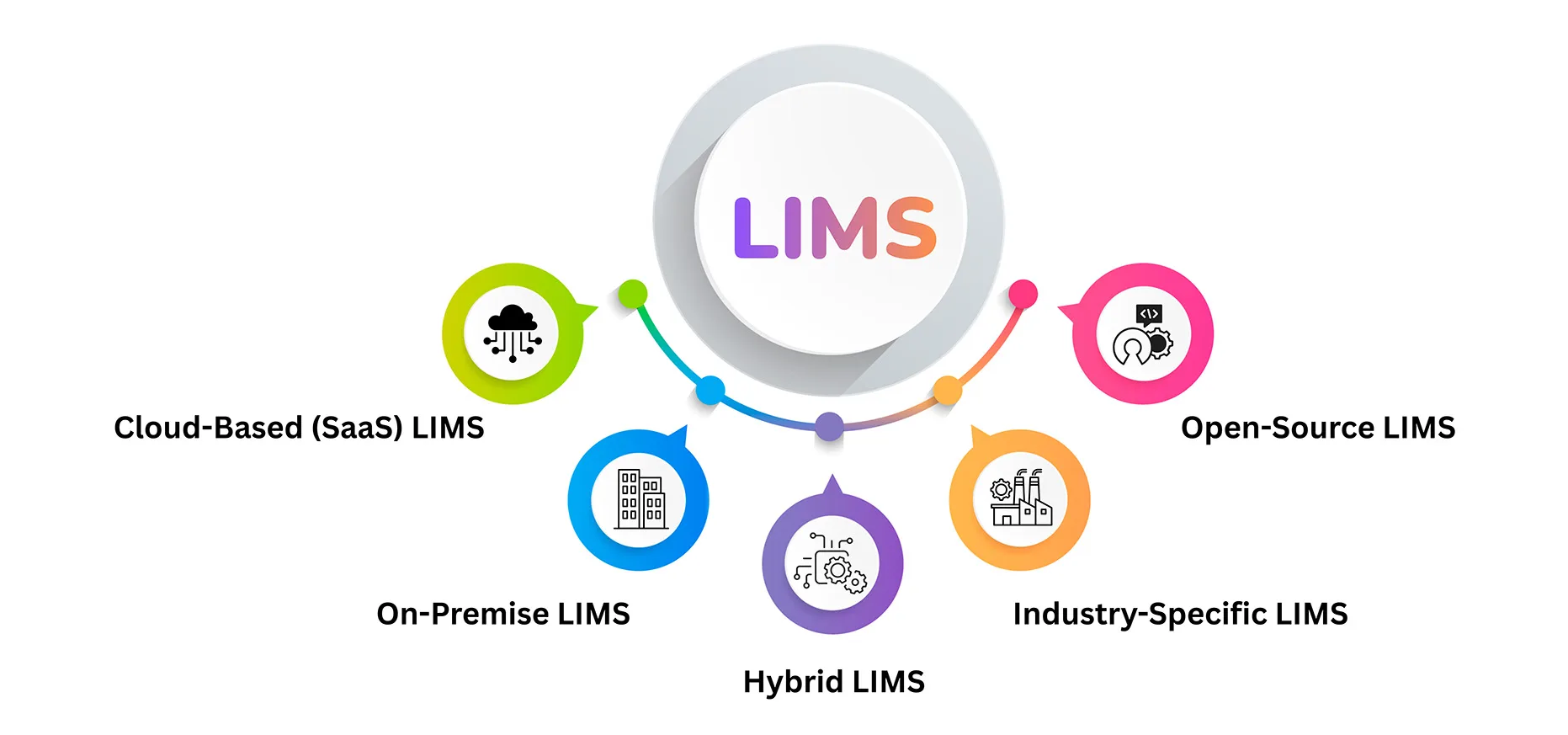
Types of LIMS Solutions
LIMS solutions come in various forms, each with distinct characteristics, advantages, and ideal use cases.
1. Cloud-Based (SaaS) LIMS
Cloud-based LIMS delivered as Software-as-a-Service runs entirely on the vendor's cloud infrastructure. Users access the system through web browsers without installing software or maintaining servers. Advantages include lower upfront costs with no hardware or infrastructure investment required, rapid deployment with implementation in weeks rather than months, automatic updates keeping you on the latest version without manual upgrades, scalability to easily add users or capacity as needs grow, accessibility from anywhere with internet connection, reduced IT burden as vendor handles maintenance, backups, and security, and predictable costs through subscription-based pricing. Considerations include internet dependency for access, data stored on the vendor's servers, less customisation flexibility than on-premise solutions, and ongoing subscription costs. Cloud-based LIMS is best for small to medium-sized laboratories, organisations wanting rapid deployment, multi-location operations requiring centralised access, and laboratories with limited IT resources.
2. On-Premise LIMS
On-premise LIMS is installed and runs on the laboratory's own servers and IT infrastructure. The organisation has complete control over the hardware, software, and data. Advantages include complete data control with all data remaining on-site behind your firewall, maximum customization with full access to modify the system to exact requirements, no internet dependency as the system operates independently of external connectivity, integration flexibility for easier connection with legacy on-premise systems, one-time licensing allowing you to pay upfront rather than recurring subscriptions, and regulatory comfort as some industries prefer on-premise for compliance. Considerations include high upfront capital investment in hardware and licenses, requirement for dedicated IT staff for maintenance and support, longer implementation timelines typically taking 6-12 months, manual upgrade processes, disaster recovery as the organization's responsibility, and limited remote access without additional infrastructure. On-premise LIMS is best for large laboratories with established IT departments, organizations with strict data sovereignty requirements, highly regulated environments with on-premise mandates, laboratories with significant customization needs, and organizations with existing IT infrastructure investments.
3. Hybrid LIMS
Hybrid LIMS combines elements of both cloud and on-premise deployments, offering flexibility in where different components of the system reside. Advantages include a balanced approach keeping sensitive data on-premise while using cloud for other functions, flexibility to choose deployment model based on specific requirements, scalability leveraging cloud for expansion while maintaining core on-premise systems, risk mitigation reducing dependency on single infrastructure model, and gradual migration transitioning from on-premise to cloud over time. Considerations include more complex architecture to manage, requirements for both on-premise IT resources and cloud management, integration between environments that must be maintained, and potentially higher overall costs. Hybrid LIMS is best for organizations transitioning from on-premise to cloud, laboratories with mixed data sensitivity requirements, multi-national corporations with varied regulatory requirements, and organizations wanting redundancy and business continuity.
4. Industry-Specific LIMS
Industry-specific LIMS solutions are pre-configured and optimized for specific industries with built-in workflows, templates, and compliance features tailored to that sector. Examples include clinical LIMS optimized for diagnostic laboratories, pharmaceutical LIMS that is GMP-compliant with stability study features, environmental LIMS offering EPA method compliance and field sampling, food safety LIMS providing HACCP support and allergen management, cannabis LIMS ensuring state compliance and potency testing, and forensic LIMS managing chain of custody and evidence. Advantages include faster implementation with pre-built workflows reducing configuration time, industry best practices built-in for compliance and methodology, lower total cost due to less customization required, regulatory alignment designed for specific regulations, and industry expertise as the vendor understands unique requirements. Considerations include potentially lacking flexibility for unique or evolving processes, vendor lock-in to industry-specific features, and possibly including features you don't need that increase cost. Industry-specific LIMS is best for laboratories with standard industry workflows, organizations new to LIMS seeking proven solutions, regulated environments requiring specific compliance features, and laboratories without resources for extensive customization.
5. Open-Source LIMS
Open-source LIMS platforms have publicly available source code that organizations can download, modify, and implement freely. Popular examples include Bika LIMS/Senaite, LabKey Server, and OpenELIS. Advantages include no licensing fees as it's free to download and use, complete customization with full access to source code, community support from active user and developer communities, transparency allowing you to review and audit all code, and avoiding vendor lock-in as you're not dependent on a single vendor. Considerations include requiring significant technical expertise to implement and maintain, limited official support requiring reliance on community or paid consultants, security and compliance responsibility falling on the organization, potentially lacking polish and features of commercial solutions, and integration and customization costs that can exceed commercial LIMS. Open-source LIMS is best for organizations with strong in-house development capabilities, academic institutions with technical resources, budget-constrained laboratories willing to invest time, organizations with unique requirements requiring extensive customization, and laboratories wanting complete control over their system.
Additional LIMS Types
Other LIMS categories include configurable versus customizable LIMS, where configurable systems can be adapted through built-in configuration tools without modifying underlying code, while customizable LIMS require code-level modifications for changes beyond standard configuration options. Integrated laboratory platforms combine LIMS with other laboratory informatics systems like ELN, SDMS, and LES in a unified solution. Mobile-first LIMS are designed with mobile devices as a primary interface, featuring native mobile apps, touch-optimized interfaces, offline data collection, and mobile barcode scanning.
LIMS Implementation: What to Expect
Successfully implementing a LIMS requires careful planning, dedicated resources, and realistic expectations. Here's a comprehensive guide to the implementation journey.
Implementation Timeline
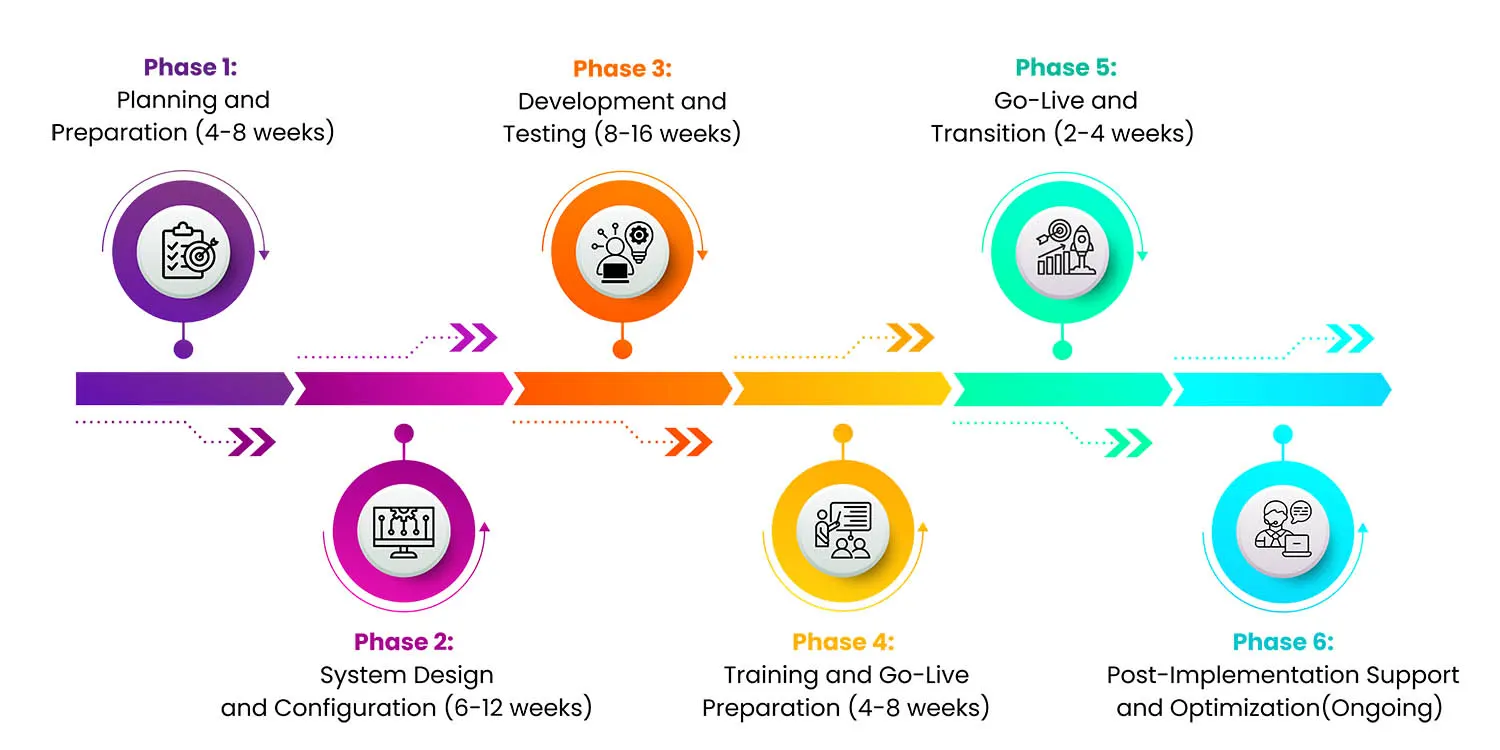
Typical timelines vary by deployment type. Cloud-based LIMS typically require 2-4 months for basic implementation. On-premise LIMS need 6-12 months for full deployment. Complex enterprise LIMS may take 12-24+ months for multi-site rollout.
Phase 1: Planning and Preparation (4-8 weeks)
Project initiation establishes a project team with defined roles and responsibilities, defines clear project scope and objectives, identifies success criteria and key performance indicators, develops a detailed project plan with milestones, and allocates budget and resources. Requirements gathering documents current workflows and processes, identifies pain points and improvement opportunities, defines must-have versus nice-to-have features, gathers input from all stakeholder groups, documents regulatory and compliance requirements, and maps out integration points with existing systems.
Key deliverables include project charter and governance structure, detailed requirements specification, risk assessment and mitigation plan, communication plan, and resource allocation plan.
Phase 2: System Design and Configuration (6-12 weeks)
System architecture designs database structure, plans network infrastructure for on-premise solutions, configures security and access controls, sets up development and testing environments, and plans disaster recovery and backup strategies. Workflow configuration maps and configures laboratory workflows, sets up sample types and test methods, configures approval chains and notifications, designs custom forms and data entry screens, creates report templates, and configures calculations and formulas.
Phase 3: Development and Testing (8-16 weeks)
System configuration builds configured workflows in the system, develops custom reports and dashboards, creates user roles and permissions, sets up validation rules and business logic, configures instrument interfaces, and builds integration connections. Data migration extracts data from legacy systems, cleans and transforms data, loads into LIMS, validates data accuracy and completeness, reconciles discrepancies, and plans for historical data access.
Testing includes unit testing to test individual components and features, integration testing to verify interfaces and data flow between systems, user acceptance testing for end users to validate the system meets requirements, performance testing to ensure the system handles expected volume and load, security testing to verify access controls and data protection, and compliance testing to document that regulatory requirements are met.
Phase 4: Training and Go-Live Preparation (4-8 weeks)
The training program includes administrator training for system configuration and maintenance, end-user training for day-to-day operations by role, management training for reporting and dashboards, and train-the-trainer sessions to build internal expertise. Hands-on practice with realistic scenarios, creation of training videos and reference materials are essential components.
Go-live preparation conducts readiness assessment, finalizes cutover plan and schedule, plans for parallel operations if applicable, prepares rollback procedures, sets up support hotline and escalation procedures, communicates go-live timeline to all stakeholders, and conducts final system validation. A pilot program is recommended, selecting limited scope or department for initial rollout, operating in parallel with legacy system, gathering feedback and refining processes, resolving issues before full deployment, and building confidence and success stories.
Phase 5: Go-Live and Transition (2-4 weeks)
Cutover activities include final data synchronization from legacy systems, switching from test to production environment, activating user accounts, beginning live operations, and providing on-site support during initial period. The hypercare period provides extended support hours with 24/7 availability if needed, rapid response to issues, daily check-ins with users, system performance monitoring, quick resolution of problems, and capturing lessons learned.
Phase 6: Post-Implementation Support and Optimization (Ongoing)
Stabilization during the first 3 months continues elevated support levels, addresses issues and user concerns, fine-tunes workflows based on real usage, provides refresher training as needed, and monitors adoption and usage metrics. Optimization analyzes performance data, identifies opportunities for improvement, implements enhancements and refinements, expands functionality incrementally, and rolls out to additional sites or departments.
Critical Success Factors
Executive sponsorship provides strong leadership support essential for securing resources, making decisions, and driving organizational change. A cross-functional team includes representatives from all stakeholder groups including laboratory staff, IT, quality, management, and end users. Realistic scope starts with core functionality and expands over time, avoiding trying to implement everything at once. Change management addresses the human side of implementation through communication, training, and support for users adapting to new processes. A vendor partnership means choosing a vendor committed to your success with responsive support and deep product expertise. Adequate resources dedicate sufficient time, budget, and personnel, recognizing implementation is not a side project. Testing and validation thoroughly test before go-live, as issues found in testing are much cheaper to fix than in production. Documentation ensures comprehensive documentation for sustainable operations and knowledge transfer. Flexibility means being prepared to adjust the plan as you learn, since implementation rarely goes exactly as initially planned. Celebrating success recognizes milestones and team contributions to maintain momentum and morale.
How to Choose the Right LIMS Software
Selecting the optimal LIMS for your laboratory is a critical decision that will impact operations for years to come.
Step 1: Assess Your Needs
Current state analysis documents existing workflows and processes, identifies pain points and inefficiencies, assesses current technology landscape, evaluates regulatory requirements, and determines budget constraints. Future state vision defines your ideal laboratory operations, identifies strategic growth plans, considers future regulatory changes, plans for technology evolution, and assesses scalability needs.
Requirements definition creates a comprehensive requirements document covering functional requirements describing what the system must do, technical requirements for integration, security, and performance, regulatory requirements for compliance mandates, usability requirements for user experience expectations, and business requirements for ROI and cost constraints. Prioritization categorizes requirements as must-have non-negotiables and deal-breakers, should-have important features with some flexibility, and nice-to-have desirable but not essential features.
Step 2: Research and Identify Vendors
Market research consults industry publications and analyst reports from Gartner and Forrester, attends professional associations and conferences, seeks peer recommendations through networking, reviews online reviews and comparison sites, and explores industry-specific vendor directories. Initial vendor lists create a long list of 8-12 potential vendors based on industry specialization matching your laboratory type, deployment model preferences for cloud versus on-premise, geographic presence and support capabilities, company size and stability, and customer base and market presence.
Step 3: Issue RFP and Evaluate Responses
For formal selection, issue a detailed Request for Proposal to shortlisted vendors, typically 3-5. Include company background describing your laboratory, operations, and strategic goals, detailed requirements covering complete functional and technical specifications, evaluation criteria explaining how proposals will be scored, implementation timeline with expected go-live date, budget parameters with realistic cost expectations, proposal format using standardized structure for easy comparison, demonstration requirements specifying specific scenarios to present, reference requests for customer contacts in similar situations, and contract terms outlining key commercial terms and conditions.
Initial screening reviews proposals against must-have requirements and eliminates vendors who cannot meet critical needs. Detailed evaluation creates scoring matrices covering functionality worth 30-40% weight assessing how well it meets requirements, technology worth 15-20% weight evaluating architecture, security, performance, and integrations, usability worth 10-15% weight examining user interface, ease of use, and mobile capabilities, vendor worth 15-20% weight assessing company stability, support quality, and industry expertise, cost worth 15-20% weight analyzing total cost of ownership and pricing model transparency, and implementation worth 10-15% weight evaluating methodology, timeline, and resource requirements.
Step 4: Conduct Product Demonstrations
Preparation defines specific scenarios reflecting your workflows, requires vendors to demonstrate with your requirements, invites broad stakeholder representation, allows 2-4 hours per vendor, and schedules question and answer time. Demonstration scenarios request vendors demonstrate sample login and accessioning, typical testing workflow from start to finish, report generation and delivery, query and data analysis capabilities, system administration and configuration, mobile capabilities if relevant, integration examples, and compliance and audit trail features.
Evaluation criteria assess ease of use and intuitive navigation, alignment with your workflows, flexibility and configurability, system performance and responsiveness, visual appeal and modern interface, and vendor knowledge and expertise demonstrated. Red flags include vague responses to specific questions, overreliance on customization for everything, heavy reliance on future features on the roadmap, inability to demonstrate key requirements, and overly complex or cumbersome workflows.
Step 5: Total Cost of Ownership Analysis
Calculate complete costs over 5 years. Cloud-based LIMS costs include annual subscription fees, implementation and configuration services, data migration, training, ongoing support often included, customization and enhancements, integration development, and total 5-year TCO. On-premise LIMS costs include software licenses perpetual or term, annual maintenance fees typically 15-20% of license cost, hardware and infrastructure, implementation and configuration services, data migration, training, IT staff for system administration, upgrades and updates, customization and enhancements, integration development, facilities costs for power, cooling, and space, and total 5-year TCO.
Hidden costs to consider include additional user licenses as you grow, optional modules not included in base price, premium support or SLAs, professional services beyond initial implementation, change management and organizational costs, and opportunity cost during implementation period.
Making the Decision
Final evaluation compiles all information including demonstration scores, reference check summaries, TCO analysis, POC results if conducted, contract terms, implementation approach, and vendor responsiveness throughout process. The decision meeting convenes the selection committee to review evaluation summary, discuss trade-offs between finalists, address any remaining concerns, reach consensus or vote, and document decision rationale. Announcement notifies the selected vendor, provides feedback to unsuccessful vendors as professional courtesy, communicates decision internally with reasons, and begins contract execution and project kickoff.
Key Selection Criteria Checklist
Functionality should meet 100% of must-have requirements and at least 80% of should-have requirements, include industry-specific features and compliance built-in, be configurable to your unique workflows, offer comprehensive reporting and analytics, and provide mobile capabilities if needed. Technology should have modern web-based architecture, proven integration capabilities with your systems, strong security features and compliance, scalability to support growth, reliable performance under load, and regular updates and innovation. Usability should feature intuitive easy-to-learn interface, positive user feedback from demonstrations, minimal clicks to complete common tasks, customizable dashboards and views, and mobile-friendly design.
Vendor criteria include stable company with strong financial position, deep experience in your industry, large satisfied customer base, positive reference checks, responsive knowledgeable sales and pre-sales team, strong implementation methodology, and excellent support reputation. Cost should be within budget parameters, have transparent predictable pricing model, reasonable TCO compared to alternatives, clear ROI path, and no significant hidden costs identified. Implementation should have realistic timeline aligned with your needs, proven implementation methodology, experienced implementation team, comprehensive training program, and adequate post-go-live support.
Future Trends in LIMS Technology
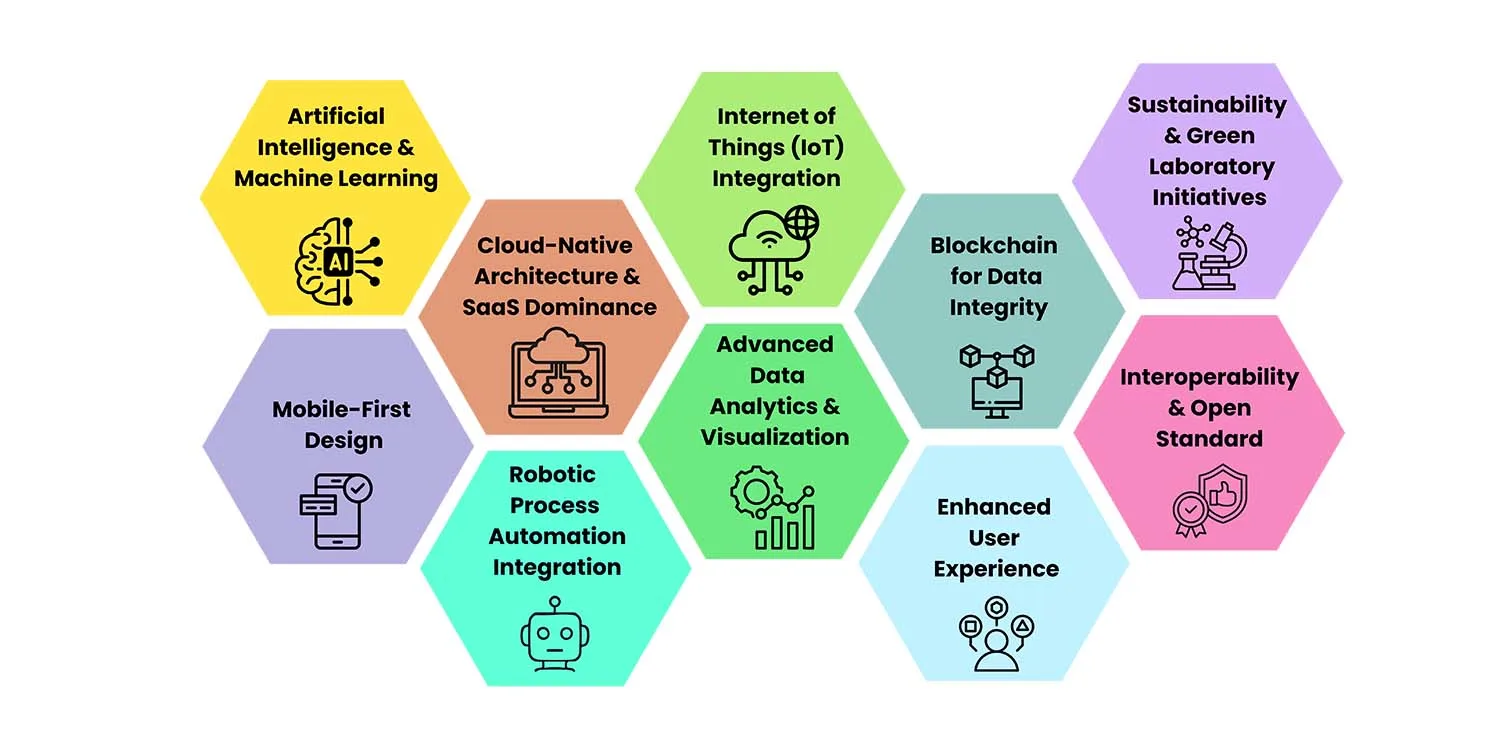
The laboratory informatics landscape is rapidly evolving, driven by advances in cloud computing, artificial intelligence, and changing user expectations.
1. Artificial Intelligence and Machine Learning
Current applications include automated data review and anomaly detection, predictive maintenance for instruments, intelligent sample routing and prioritization, and result prediction based on historical patterns. Future developments will bring AI-powered quality control with machine learning algorithms that learn normal patterns and automatically flag anomalies with greater accuracy than traditional control charts, predictive analytics forecasting testing demand, resource requirements, and potential bottlenecks before they occur, automated method development with AI suggesting optimal testing methodologies based on sample characteristics and desired outcomes, natural language interfaces allowing users to query data and generate reports using conversational AI, and image recognition for automated analysis of visual results including colony counts, chromatograms, and microscopy.
Impact: Laboratories will shift from reactive to proactive operations, with AI handling routine decisions and flagging only exceptions for human review, dramatically improving efficiency while enhancing quality.
2. Cloud-Native Architecture and SaaS Dominance
The trend shows accelerating migration from on-premise to cloud-based LIMS, with cloud-native architectures becoming the standard. Advantages driving adoption include lower total cost of ownership, faster deployment and updates, better scalability and flexibility, enhanced disaster recovery, reduced IT burden, and improved accessibility for remote and mobile users. By 2030, industry analysts predict 70-80% of new LIMS deployments will be cloud-based, with on-premise systems reserved for highly specialized or security-sensitive environments.
3. Internet of Things (IoT) Integration
The connected laboratory will see LIMS serve as the central hub for IoT-enabled laboratory devices. Smart instruments will continuously monitor instrument status, performance, and maintenance needs. Environmental sensors will provide real-time tracking of temperature, humidity, and other critical conditions. Smart storage will enable automated inventory tracking using RFID and weight sensors. Wearable devices will integrate with smart glasses or watches for hands-free operation. Benefits include proactive maintenance reducing downtime, automated documentation of environmental conditions, real-time alerts for out-of-spec conditions, elimination of manual equipment logs, and improved data integrity with automated capture.
4. Mobile-First Design
LIMS interfaces are being redesigned with mobile devices as the primary consideration, not as an afterthought. Mobile capabilities include field sampling for complete sample collection workflows on tablets or smartphones, barcode scanning using device cameras for sample identification, photo documentation capturing visual evidence directly into LIMS, real-time notifications through push alerts for urgent actions needed, offline operation for collecting data without connectivity and syncing when available, and voice input for hands-free data entry and navigation. Impact: Mobile-first design will make LIMS more accessible, intuitive, and efficient, particularly for field operations and manufacturing floor quality control.
5. Advanced Data Analytics and Visualization
Beyond basic reporting, LIMS will incorporate sophisticated analytics tools including interactive dashboards for real-time visualization of KPIs with drill-down capabilities, predictive analytics for forecasting trends and identifying patterns, statistical process control with advanced control charting and capability analysis, business intelligence integration seamlessly connecting with tools like Power BI, Tableau, and Qlik, and big data analytics for analyzing massive datasets for insights. Self-service analytics will enable laboratory staff and management to create their own reports and analyses without IT involvement, democratizing data access.
6. Blockchain for Data Integrity
Application of blockchain technology will ensure immutable audit trails and data integrity through chain of custody providing unalterable records of sample handling, data authenticity offering cryptographic proof that data hasn't been tampered with, multi-party collaboration enabling secure data sharing across organizations, regulatory compliance meeting 21 CFR Part 11 and data integrity requirements, and supply chain transparency providing end-to-end traceability for materials and samples. Adoption timeline: While still emerging, blockchain integration is expected to become standard in highly regulated industries like pharmaceutical and forensic within 5-7 years.
7. Robotic Process Automation Integration
Laboratory automation will see LIMS increasingly orchestrate robotic systems for automated sample handling with robots moving samples through workflows, liquid handling systems integrating with automated pipetting and dispensing, sample prep automation coordinating extraction, digestion, and dilution robots, high-throughput screening managing automated testing platforms, and automated storage and retrieval interfacing with robotic freezer systems. Software robots through RPA bots will handle repetitive digital tasks including data transfer between systems, report generation and distribution, invoice processing, and compliance documentation. Impact: Seamless human-robot collaboration will dramatically increase laboratory capacity while reducing errors and costs.
8. Enhanced User Experience
Consumer-grade interfaces will see LIMS adopt design principles from consumer applications including intuitive navigation with Netflix-like simplicity, personalization with interfaces that adapt to individual user preferences and roles, contextual help with smart assistance appearing when needed, minimal training requirements through self-evident functionality, and responsive design seamlessly adapting to any device or screen size. Voice and gesture control will enable voice commands for hands-free operation in laboratory settings, gesture-based navigation for sterile environments, and virtual keyboard alternatives for gloved users.
9. Interoperability and Open Standards
Breaking down silos, future LIMS will emphasize seamless integration through API-first architecture with all functionality accessible via modern REST APIs, open standards supporting FHIR, HL7, ASTM, AnIML, and other industry standards, microservices with modular architecture allowing best-of-breed component selection, pre-built connectors for out-of-box integration with common systems like ERP, CRM, and instruments, and data federation providing unified view across multiple data sources without physically moving data. The laboratory ecosystem will see LIMS serve as the orchestration layer connecting Electronic Laboratory Notebooks, Scientific Data Management Systems, Chromatography Data Systems, Enterprise Resource Planning systems, Customer Relationship Management platforms, and Manufacturing Execution Systems.
10. Sustainability and Green Laboratory Initiatives
Environmental tracking will help laboratories reduce environmental impact through energy consumption monitoring tracking instrument and facility usage, waste tracking documenting chemical and hazardous waste generation, carbon footprint calculation measuring and reporting environmental impact, sustainability reporting with automated generation of environmental performance metrics, resource optimization identifying opportunities to reduce consumption, and green chemistry supporting selection of environmentally-friendly methods. The paperless laboratory continues the drive toward complete elimination of paper through digital signatures, electronic records, and mobile workflows.
Frequently Asked Questions About LIMS
What does LIMS stand for?
LIMS stands for Laboratory Information Management System. It is specialized software designed to manage laboratory operations, samples, data, workflows, and compliance in a centralized digital platform.What is the difference between LIMS and LIS?
LIMS (Laboratory Information Management System) is designed for research, industrial, environmental, and quality control laboratories, while LIS (Laboratory Information System) is specifically designed for clinical diagnostic laboratories in healthcare settings. LIMS focuses on sample tracking, workflow management, and regulatory compliance for diverse testing types, while LIS emphasizes patient demographics, clinical result reporting, and integration with hospital information systems.How much does LIMS cost?
LIMS costs vary based on size and deployment type. Small cloud-based LIMS systems cost $10,000-50,000 annually. Medium implementations run $50,000-150,000 per year. Large on-premise solutions start at $100,000-500,000+ for initial licenses plus 15-20% annual maintenance. Total cost of ownership over 5 years includes software, implementation services ($20,000-1,000,000+), training, data migration, and ongoing support.How long does LIMS implementation take?
Implementation timelines vary by deployment type and complexity. Cloud-based LIMS typically take 2-4 months for basic implementations. On-premise LIMS require 6-12 months for full deployment. Complex enterprise LIMS need 12-24+ months for multi-site rollouts. Factors affecting timeline include customization requirements, data migration complexity, integration needs, and organizational readiness.What industries use LIMS?
LIMS is used across virtually every industry that conducts laboratory testing including pharmaceutical and biotechnology, clinical and diagnostic laboratories, environmental testing, food and beverage safety, cannabis testing, oil and gas/petrochemical, agriculture and agribusiness, chemical manufacturing, water and wastewater utilities, contract testing laboratories, academic and research institutions, cosmetics and personal care, mining and metals, forensic laboratories, and veterinary diagnostics.Can LIMS integrate with laboratory instruments?
Yes, modern LIMS solutions offer extensive instrument integration capabilities. Most LIMS can connect with common laboratory instruments including HPLC, mass spectrometers, PCR machines, spectrophotometers, balances, pH meters, and more. Integration can be achieved through direct bi-directional communication protocols, middleware solutions, file-based import/export, OPC standards, and vendor-specific APIs.Is LIMS suitable for small laboratories?
Absolutely. Cloud-based LIMS solutions have made laboratory management software accessible and affordable for laboratories of all sizes. Small laboratories benefit from lower upfront costs with subscription pricing, rapid deployment in weeks rather than months, minimal IT infrastructure requirements, scalability to grow with the laboratory, professional reporting and compliance capabilities, and improved efficiency even with limited staff.What is the ROI of implementing LIMS?
Return on investment from LIMS typically includes quantifiable benefits such as labor cost reduction with 20-40% efficiency gains, reduced material waste with 10-20% savings, decreased rework and retesting with 50-80% reduction in errors, faster turnaround enabling revenue growth with 30-50% faster reporting, and reduced IT costs for cloud solutions with 40-60% infrastructure savings. Strategic benefits include improved client satisfaction and retention, competitive advantage through certifications, reduced regulatory risk and penalties, better decision-making through data access, and scalability supporting business growth. Most organizations achieve positive ROI within 6-18 months, with total ROI of 200-400% over five years.How secure is LIMS data?
Modern LIMS solutions employ multiple layers of security including encryption with data encrypted both in transit using TLS/SSL and at rest, access controls with role-based permissions restricting functionality and data access, authentication with multi-factor authentication options, audit trails providing complete logging of all system activities, data backup with automated redundant backups and disaster recovery, compliance with security standards including SOC 2, ISO 27001, and HIPAA, physical security as cloud providers maintain secure monitored data centers, and regular testing through penetration testing and security audits.
Can LIMS handle multiple locations?
Yes, LIMS solutions can support multi-site operations through centralized databases providing single source of truth accessible from all locations, site-specific configurations customizing workflows for each location while maintaining consistency, cross-site visibility enabling management to view operations across all locations, sample transfer tracking samples moving between sites, consolidated reporting aggregating data across the organization, and role-based access providing appropriate permissions for each site and user.
Do I need IT staff to manage LIMS?
Requirements depend on the deployment model. Cloud-based LIMS requires minimal IT with the vendor handling infrastructure, security, backups, and updates, and laboratory staff typically managing day-to-day administration with vendor support for complex issues. On-premise LIMS requires dedicated IT staff or resources for server management, database administration, security, backups, and troubleshooting. Most laboratories can successfully operate cloud-based LIMS without dedicated IT staff, making it ideal for smaller organizations.
What regulatory standards does LIMS support?
Modern LIMS solutions support compliance with major regulatory standards including FDA 21 CFR Part 11 for electronic records and signatures, ISO/IEC 17025 for testing and calibration laboratory competence, GLP (Good Laboratory Practice) for non-clinical laboratory studies, GMP (Good Manufacturing Practice) for pharmaceutical manufacturing, CLIA (Clinical Laboratory Improvement Amendments), CAP (College of American Pathologists) standards, HIPAA for healthcare data privacy in clinical labs, EPA methods for environmental testing compliance, and HACCP for food safety management.
Can LIMS be accessed remotely?
Yes, modern LIMS solutions, particularly cloud-based systems, provide remote access through web browsers for access from any device with internet connectivity, mobile apps with dedicated applications for smartphones and tablets, virtual private networks for secure access to on-premise systems, and client portals allowing clients to submit samples and view results remotely. Remote access enables work from home, field sampling, multi-site operations, and client self-service while maintaining security and compliance.
What training is required for LIMS users?
Training requirements vary by role and system complexity. End users like laboratory technicians need 4-8 hours initial training on core workflows, hands-on practice with realistic scenarios, quick reference guides for common tasks, and ongoing refresher training as needed. Power users including supervisors and analysts need 1-2 days comprehensive training covering advanced features and reporting, and troubleshooting common issues. System administrators require 2-5 days technical training covering system configuration and maintenance, user management and security, and report development. Note:- This can vary according to the end user requirements and specific workflows.
Can I try LIMS before purchasing?
Many vendors offer evaluation options including free trials with 14-30 day access to test environments, product demonstrations with customized demos showing your workflows, proof of concept with limited implementation to test with your data, sandbox environments to practice with sample data, and reference visits to tour customer sites using the system. It's highly recommended to thoroughly evaluate LIMS through demonstrations and hands-on testing before making a purchasing decision.
What happens to my data if I change LIMS vendors?
Data ownership and portability are critical considerations. You own your data, which should be clearly stated in your contract. LIMS should provide tools to export data in standard formats like CSV, XML, and PDF. Transition assistance with many contracts includes provisions for vendor support during transition. Archive access maintains read-only access to historical data if needed for compliance. Standard formats using industry-standard data formats facilitates migration. Before signing a contract, verify your rights to data export at any time, available data formats for export, transition support included or available, historical data retention options, and no penalties or restrictions on data portability.
Ready to Transform Your Laboratory with LIMS?
Implementing a Laboratory Information Management System is a significant step toward operational excellence, improved quality, and regulatory confidence. Whether you're running a small testing laboratory or managing a complex multi-site operation, the right LIMS can deliver transformative benefits.
Next Steps to Consider
- Assess Your Needs: Document your current challenges, workflows, and requirements
- Engage Stakeholders: Involve all groups who will be affected by LIMS
- Research Options: Explore vendors specializing in your industry and laboratory type
- Request Demonstrations: See systems in action with your specific workflows.
- Check References: Talk to laboratories similar to yours about their experiences
- Evaluate Total Cost: Consider 5-year cost of ownership, not just initial price
- Plan for Success: Allocate adequate resources for implementation and change management
Why Choose Revol LIMS?
Revol LIMS is a modern, cloud-based laboratory information management system designed to streamline operations, ensure compliance, and drive efficiency for laboratories across industries. With intuitive workflows, powerful integrations, and industry-leading support, Revol LIMS helps laboratories of all sizes achieve their quality and productivity goals.
Key Advantages:
- Rapid deployment - Go live in weeks, not months
- Intuitive interface - Minimal training required for high user adoption
- Industry-specific solutions - Pre-configured for your laboratory type
- Comprehensive compliance - Built-in support for FDA, ISO, EPA, and other standards
- Flexible and scalable - Grows with your laboratory
- Exceptional support - Dedicated team committed to your success
Take Action Today: Schedule a demo to see Revol LIMS in action with workflows customized to your laboratory. Transform your laboratory operations with Revol LIMS - where innovation meets reliability, and efficiency meets compliance.
About This Guide
Author: Revol LIMS Expert TeamLast Updated: January 2026
Version: 2.0
This comprehensive guide to Laboratory Information Management Systems represents the collective expertise of laboratory professionals, informatics specialists, and industry experts with over 15 years of combined experience in laboratory management and LIMS implementation. We are committed to providing accurate, current, and actionable information to help laboratories make informed decisions about LIMS technology.
Have questions or feedback? Contact our team at marketing@revollims.com