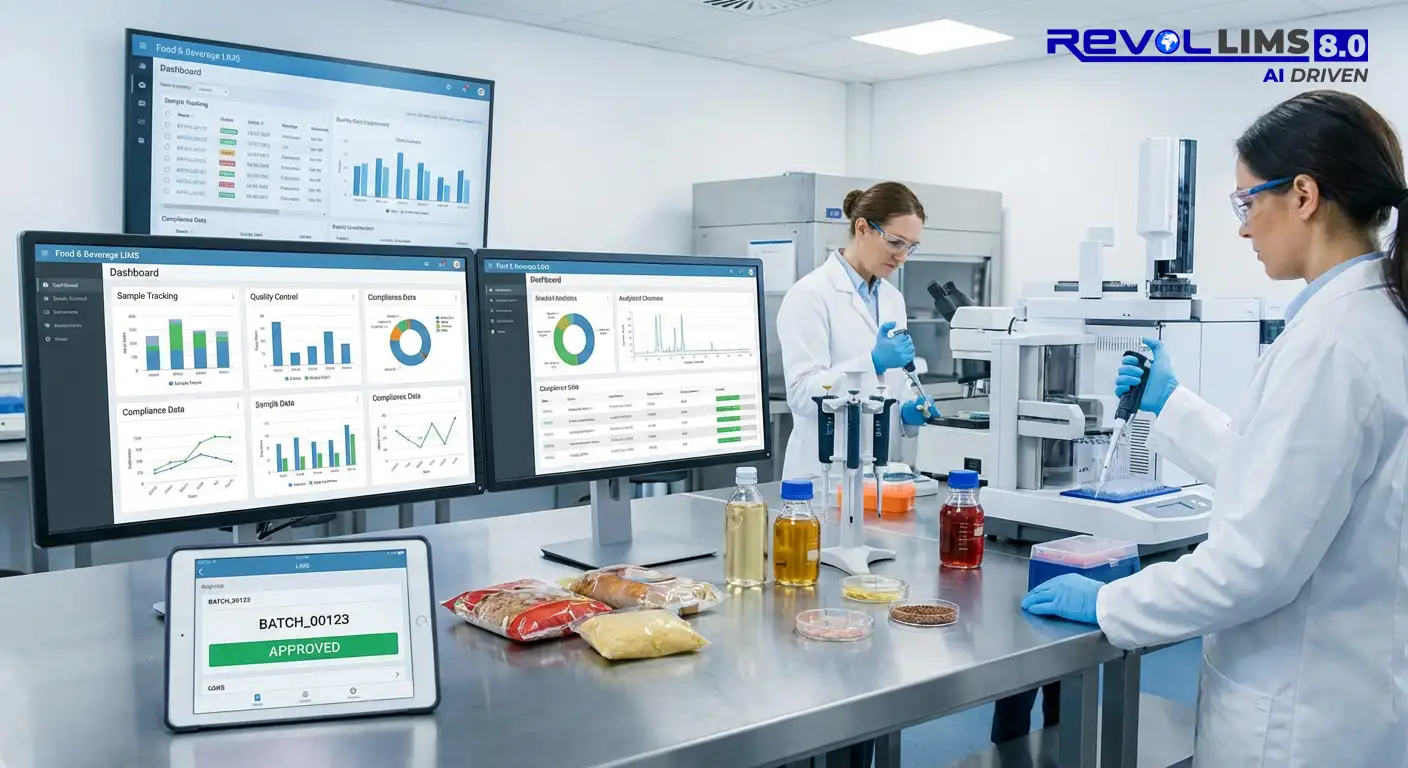
Enhances and Automates your Pharmaceutical Laboratories Data Management and Quality
Pharma LIMS is an all-in-one solution that simplifies and optimizes lab processes, ensuring better data integrity, compliance, and operational efficiency. Its automation capabilities and real-time insights help pharmaceutical companies improve quality control, reduce costs, and enhance decision-making, all while ensuring the highest regulatory standards.
Key Features of Pharma LIMS:
Sample Management
 Efficient Tracking: Pharma LIMS enables real-time tracking of samples from receipt to disposal, reducing the risk of sample loss and ensuring precise record-keeping.
Efficient Tracking: Pharma LIMS enables real-time tracking of samples from receipt to disposal, reducing the risk of sample loss and ensuring precise record-keeping.
 Automation: The system automates data entry and sample processing, minimizing manual errors and boosting efficiency.
Automation: The system automates data entry and sample processing, minimizing manual errors and boosting efficiency.
 Batch and Lot Management: Easily track pharmaceutical batches and lots for improved traceability and quality control.
Batch and Lot Management: Easily track pharmaceutical batches and lots for improved traceability and quality control.
Regulatory Compliance
 21 CFR Part 11 Compliance: Pharma LIMS meets FDA requirements for electronic records and signatures, providing complete audit trails for all actions.
21 CFR Part 11 Compliance: Pharma LIMS meets FDA requirements for electronic records and signatures, providing complete audit trails for all actions.
 Good Laboratory Practices (GLP) & GMP Compliance: The system adheres to global standards, ensuring that your laboratory operations align with industry regulations.
Good Laboratory Practices (GLP) & GMP Compliance: The system adheres to global standards, ensuring that your laboratory operations align with industry regulations.
 Automated Reporting: Reports are generated with minimal manual effort, ensuring compliance and accuracy during audits.
Automated Reporting: Reports are generated with minimal manual effort, ensuring compliance and accuracy during audits.
Data Integrity & Security
 Centralized Data Storage: All laboratory data is securely stored in one centralized system, offering a single point of truth for faster, more accurate decision-making.
Centralized Data Storage: All laboratory data is securely stored in one centralized system, offering a single point of truth for faster, more accurate decision-making.
 Data Security: High-level security features, including access controls and encrypted data transmission, ensure the protection of sensitive information.
Data Security: High-level security features, including access controls and encrypted data transmission, ensure the protection of sensitive information.
 Audit Trails: The system records every action taken on data, from entry to report generation, ensuring transparency and accountability.
Audit Trails: The system records every action taken on data, from entry to report generation, ensuring transparency and accountability.
Real-Time Data Access & Reporting
 Real-Time Updates: Pharmaceutical teams can access up-to-the-minute data on sample status, test results, and laboratory metrics, supporting timely decision-making.
Real-Time Updates: Pharmaceutical teams can access up-to-the-minute data on sample status, test results, and laboratory metrics, supporting timely decision-making.
 Customizable Reports: Create customized reports, including quality control results, trend analyses, and compliance checks tailored to your lab's needs. Integrated Quality Control (QC)
Customizable Reports: Create customized reports, including quality control results, trend analyses, and compliance checks tailored to your lab's needs. Integrated Quality Control (QC)
 Automated QC Checks: Pharma LIMS automates quality control processes by validating results against pre-set standards, notifying users of any deviations.
Automated QC Checks: Pharma LIMS automates quality control processes by validating results against pre-set standards, notifying users of any deviations.
 Deviation Management: The system triggers alerts and facilitates corrective actions whenever test results or processes deviate from established standards. Efficient Lab Workflow
Deviation Management: The system triggers alerts and facilitates corrective actions whenever test results or processes deviate from established standards. Efficient Lab Workflow
 Workflow Automation: Routine tasks like sample sorting, test assignments, and report generation are automated, increasing lab throughput and reducing manual work.
Workflow Automation: Routine tasks like sample sorting, test assignments, and report generation are automated, increasing lab throughput and reducing manual work.
 Lab Instrument Integration: The system integrates seamlessly with laboratory instruments, enabling automatic data entry, reducing manual input, and ensuring accuracy.
Lab Instrument Integration: The system integrates seamlessly with laboratory instruments, enabling automatic data entry, reducing manual input, and ensuring accuracy.
 Real-Time Scheduling: Scheduling lab tasks, resources, and instruments is made easier with real-time visibility into available capacity.
Real-Time Scheduling: Scheduling lab tasks, resources, and instruments is made easier with real-time visibility into available capacity.
Data Analysis & Analytics
 Trend Analysis: Pharma LIMS offers tools to analyze data trends, helping to identify potential issues early before they escalate.
Trend Analysis: Pharma LIMS offers tools to analyze data trends, helping to identify potential issues early before they escalate.
 Advanced Analytics: Built-in analytics allow labs to assess test results, evaluate product quality, and optimize processes to improve operational efficiency and reduce costs.
Advanced Analytics: Built-in analytics allow labs to assess test results, evaluate product quality, and optimize processes to improve operational efficiency and reduce costs.
Scalability and Flexibility
 Scalable for Growth: Whether you operate a small lab or a large-scale pharmaceutical company, Pharma LIMS is scalable to grow with your business needs.
Scalable for Growth: Whether you operate a small lab or a large-scale pharmaceutical company, Pharma LIMS is scalable to grow with your business needs.
 Customizable Features: The system is adaptable to suit the unique requirements of different lab types, from R&D to quality control to clinical testing.
Customizable Features: The system is adaptable to suit the unique requirements of different lab types, from R&D to quality control to clinical testing.
Cloud-Based Accessibility
 Remote Access: With a cloud-based platform, laboratory data and operations can be accessed remotely, offering flexibility, especially for multi-site pharmaceutical organizations.
Remote Access: With a cloud-based platform, laboratory data and operations can be accessed remotely, offering flexibility, especially for multi-site pharmaceutical organizations.
 Data Backup and Recovery: Cloud solutions provide automatic data backups and disaster recovery, ensuring your laboratory’s data is secure and easily recoverable.
Data Backup and Recovery: Cloud solutions provide automatic data backups and disaster recovery, ensuring your laboratory’s data is secure and easily recoverable.
Compliance Requirements
- FDA 21 CFR Part 11
- EU GMP Annex 11
- ICH Guidelines
- USP/BP/EP Standards
- WHO GMP
Key Functionalities
- Stability study management
- Comprehensive audit trail system
- Electronic signature compliance
- Instrument calibration and maintenance tracking
- Quality control and assurance workflows
- Manage staff training and competency
- Document Management